A conversational guide to a modern lab operating system
Modern labs want speed and trust at once. Scispot gives both. It unifies ELN, LIMS, and data lake. It connects instruments, people, and data. It keeps work auditable without slowing teams down.
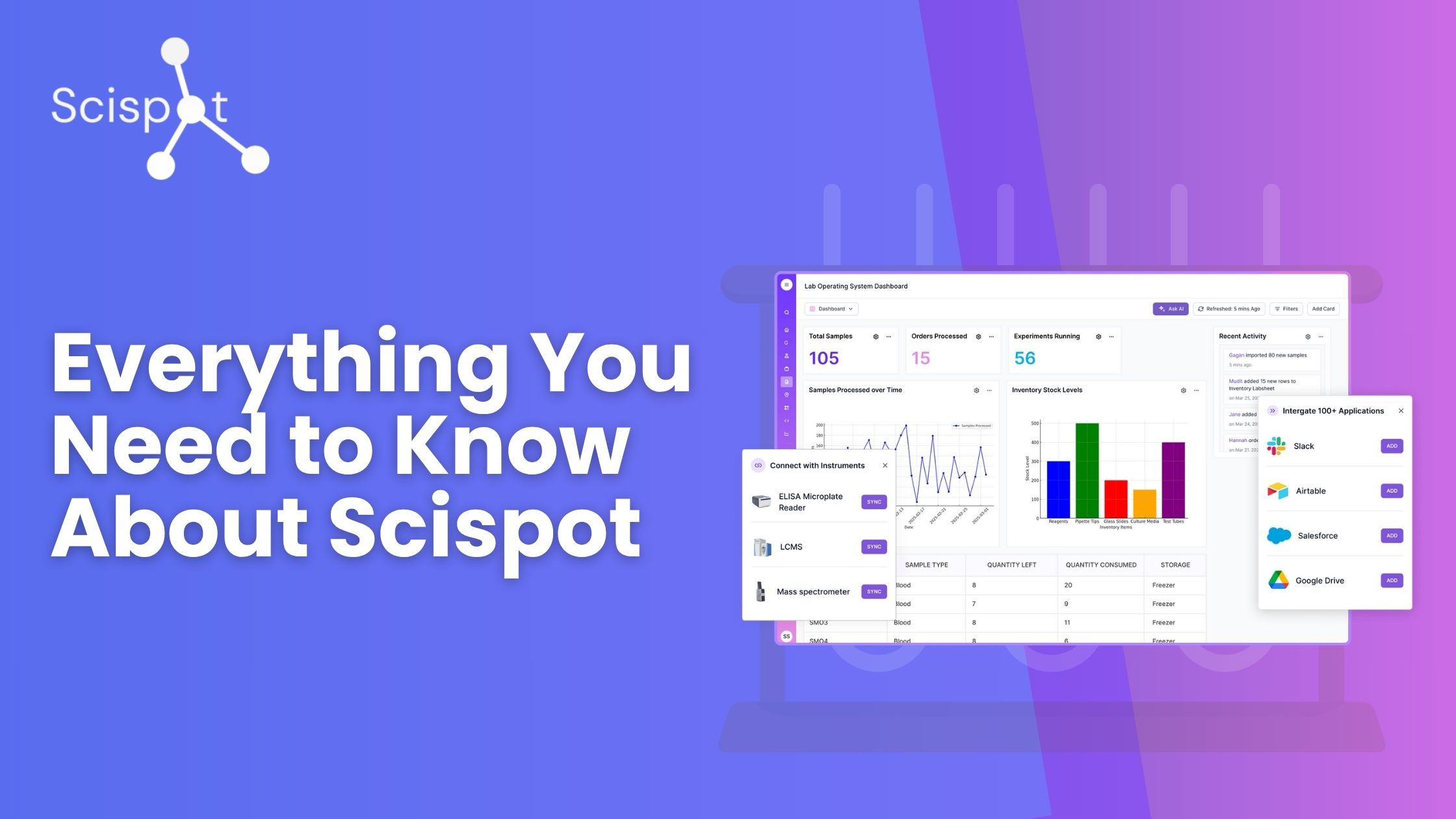
Scispot feels like your lab's operating system. You write and track work in one place. You store and shape data in another. You move through guided steps from intake to report with a clear trail.
Context: why labs switch now
Legacy tools hit a ceiling. Teams need flexible R&D and rigid clinical in one system. They are done juggling two stacks.
Audits arrive faster. Leaders want clean provenance, e‑signatures, and training gates. Loose files and binders are no longer enough.
Instrument data grows. New sequencers and PCR rigs stream files daily. Manual uploads cause drift and rework.
Knowledge turns over. Students and staff rotate. Protocols and results must stay usable by the next person.
Operations scale. Sample volumes spike. Plate maps multiply. Managers need warnings before bottlenecks form.
Internal builds stall. Homegrown apps start strong and then stall under load. Teams want a platform built for labs from day one.
Budget windows open. Funding cycles reward teams who can prove value quickly. A scoped pilot helps lock that decision in.
What Scispot is?
.png)
Labspaces hold protocols, SOPs, and experiments. They behave like a structured notebook with versioning. You can start from a template or build from scratch. Files, images, sequences, and results live together.
Labsheets are tables for samples, reagents, runs, and outcomes. They look simple but enforce structure. Columns and permissions are set once and reused. Updates can be manual, bulk, or via API.
Labflows guides technicians step by step, automating sample-centric workflows. They batch samples, assign tests, check rules, and enforce thresholds. They work like a GPS for the bench. Clear, repeatable, and hard to misuse.
A built‑in copilot (Scispot AI) helps you find what matters. Ask for the last run of a protocol. Jump to a sample's lineage. Search across Space, Sheet, and Flow with context and history.
Manifests map well plates to real items. Parent–child links stay intact. Plate views stay in sync with inventory and results. Nothing gets lost between the bench and the database.
Data in, insight out
Scispot speaks open standards. Instruments connect through a universal connector that supports common health and lab protocols. Flat files are welcome when APIs are not available. Webhooks and REST keep systems in sync without manual uploads.
Data follows an ELT path. You load first. You transform with human review. You publish to your lake or warehouse when the team is satisfied. You can always reach both the raw files and the clean sets.
Analytics sit next to the data. You can ask in plain language and get a suggested view. You can trace every step that shaped a chart or metric. When you need depth, notebooks run close to the source. Teams share code, lock versions, and avoid single‑person bottlenecks.
Search feels natural because objects are linked. Graph and vector indexing connect runs, plates, SOPs, and results. You find the right thing without hunting through folders.
Inventory that updates itself
Scispot tracks reagents, lots, and storage down to the shelf and box. You print labels and scan at the bench. When you use a material in an ELN step, stock levels drop. If a threshold hits, the right person gets an alert. Expiries and holds are visible before they cause delays.
A virtual map mirrors your freezers and rooms. Moves are logged. History is always available. You see where every vial was and where it is now.

Compliance that scales with you
Scispot supports 21 CFR Part 11 controls for e‑records and e‑signatures. It aligns with GxP, CLIA, CAP, and ISO 15189 programs. It supports GDPR and IVDR efforts and can execute BAAs for HIPAA when required.
Every change is logged. Roles and training gates control who can perform and who can sign. Validation services cover IQ, OQ, and PQ. Research work stays flexible. Clinical work stays locked and traceable.
Order intake and reporting
Orders can arrive through a portal or by API from systems you already use. Reports pull from Lab Sheets and ELN records. They include signatures and context. Delivery can be through a portal or pushed to external systems on a schedule.
If a result changes, the audit trail shows who changed it and why. Nothing is hidden. Everything is explainable.
Automation with a human gate
A status change can trigger a calculation, a script, or a request for review. Approvers can block or release the next step. Notifications reach people by email and common chat tools. Pipelines move faster, yet a human stays in the loop.
Think of it like cruise control. The system handles routine steps. You steer at key moments.

Planning and capacity
You can plan lab work on a simple timeline. You can set due dates and see the load by team or instrument. The system flags risk when a run is late or a station is over capacity. It does not drive machines. It gives clear visibility so you can act early.
Dashboards show today's runs, tomorrow's risks, and this week's completions. Managers get signal, not noise.
Scale, reliability, and data ownership
Scispot runs on large, global cloud regions. It auto‑scales for high volume and keeps strong uptime targets. A live dashboard shows connector health. If a service hiccups, rapid saves and detailed logs protect your work and support restoration.
You own your data. You can export in bulk at any time. Backups are managed by default. You can also push copies to your own cloud on your schedule. Regional residency is available when policy demands it.
Security, privacy, and sovereignty
Data is encrypted in transit and at rest. Access is role‑based and audited. Residency options support regional policies. For pilots, data can start in a default region and move later for full deployment. The platform meets privacy expectations without adding friction to daily work.
Commercials in plain language
Pricing is user‑based with volume benefits. Integrations and instruments can be added as modules. A paid pilot proves value with a fixed scope and clear deliverables. Compute is not metered in a strict way under typical use. When usage is very unusual, the team tunes the setup instead of surprising you.

Implementation and migration
You see movement quickly. The first month focuses on a clean data model, a guided flow, and one instrument feeding live data. The second month expands sheets, adds analytics views, and tunes roles. The third month locks validated steps, promotes reports, and pushes data to your lake.
Data migration is model‑first. You define entities and relationships. You import with checks. AI‑guided CSV mapping and watchers reduce manual effort. This keeps lineage clean and avoids rework later.
Change management is simple. Champions lead small wins. Short videos and checklists help new users. Most updates are no‑code. Scripts are yours to own.
Decision desk: common evaluation questions and clear answers
"Will it replace both my ELN and LIMS?" Yes. It handles protocol authoring, experiment capture, sample tracking, inventory, QC, analytics, and reporting in one place. You can run open R&D and locked clinical workflows on the same platform.
"Will integrations be a burden on our IT team?" No. The platform is API‑first and standards‑driven. The universal connector supports ASTM, HL7, and REST. Flat files are supported when needed. The onboarding team maps formats and validates feeds so data lands in the right tables from day one.
"Is the system flexible enough for creative research?" Yes. R&D can start open, add structure, and then lock down when ready. Templates reduce drift. Views and roles keep sensitive work contained.
"Can the portal and reports match our language?" Yes. Reports and portals can reflect your terms and sections. Signatures and context stay intact.
"Will it scale if our volume doubles?" Yes. Auto‑scaling handles peaks. Throughput, read/write patterns, and spike management are standard. Extremely high API traffic can be tuned with caching and batch patterns.
"Do we keep control?" Yes. You own scripts and configurations. Your team can update protocols, views, and flows without waiting on a vendor queue.

A day in the life
A lab coordinator opens a flow and scans new samples. Due dates set themselves. A capacity warning triggers a shift in assignments. Delays never start.
An instrument drops a run file into a watched folder. Scispot ingests it and runs a cleanup script. A reviewer approves with one click. Clean data posts to a master table and a copy lands in the warehouse.
A scientist asks the copilot for the last five runs that failed QC on a panel. The view appears with trend lines and notes. A small protocol tweak is drafted in Lab Spaces and tested in R&D mode. The team locks the new version for clinical use after validation.
At close, reports generate with signatures and lot numbers. A downstream system receives counts by API. Backups run, and a copy of key tables lands in the lab's own cloud. Nothing sits in an inbox.
Final word
Modern labs need clear data, smooth execution, and audit‑ready records. Scispot delivers all three. It meets you where you are and grows with your science. If you want to replace CSV chaos with a calm, connected lab, this is how you start.





.webp)
.webp)
.webp)



