Can you recommend reliable pharmaceutical systems for a new lab?
Setting up a new lab is an exciting venture. However, it comes with its own set of challenges. One of the most critical aspects is choosing reliable pharmaceutical systems. These systems ensure the safety and efficacy of medications.
Reliable systems are essential for maintaining compliance with industry standards. They help prevent medication errors and streamline operations. Secure medication systems are a key component of any successful lab.
Pharmaceutical systems should be scalable and adaptable. They need to accommodate future growth and technological advancements. Integration with existing lab equipment is also crucial.
In this guide, we will explore the best pharmaceutical systems for new labs. We will provide insights into secure medication systems and pharma solutions. Our aim is to help you make informed decisions for your lab's success.
Why Reliable Pharmaceutical Systems Matter in New Labs
Reliable pharmaceutical systems are vital for new labs. They ensure safe and effective medication management. Their role is pivotal in maintaining patient safety and compliance.
Secure medication systems help minimize the risk of errors. Errors can lead to costly mistakes and harm. Implementing a reliable system is a proactive measure against such risks.
Consider these reasons why they matter:
- Ensure compliance with industry standards
- Prevent medication errors
- Optimize lab efficiency
Beyond safety, these systems support seamless lab operations. They integrate with other lab components, creating a cohesive workflow. This integration is crucial for maintaining data accuracy and consistency.
New labs can greatly benefit from real-time monitoring features. This technology improves decision-making and operational efficiency. For a successful setup, investing in the right pharmaceutical systems is essential.
Key Features of Secure Medication Systems
Secure medication systems offer numerous features crucial to lab efficiency and safety. They provide a comprehensive approach to medication management. Effective systems integrate technology and process.
A key feature is automation, which reduces manual errors. Automated dispensing systems ensure precise dosage delivery. This leads to improved patient safety and outcomes.
Another feature is data security. Secure systems protect sensitive patient and medication data. This is vital in safeguarding against data breaches and unauthorized access.
Here are essential features to consider:
- Automated dispensing and tracking
- Robust data security and encryption
- Real-time monitoring and alerts
User-friendly interfaces also play a significant role. They ensure staff can operate systems with minimal training. This reduces the likelihood of operational mistakes.
Lastly, customizable reporting features aid in compliance. By providing detailed insights, these features support regulatory adherence. Selecting a system with these capabilities can bolster lab success.
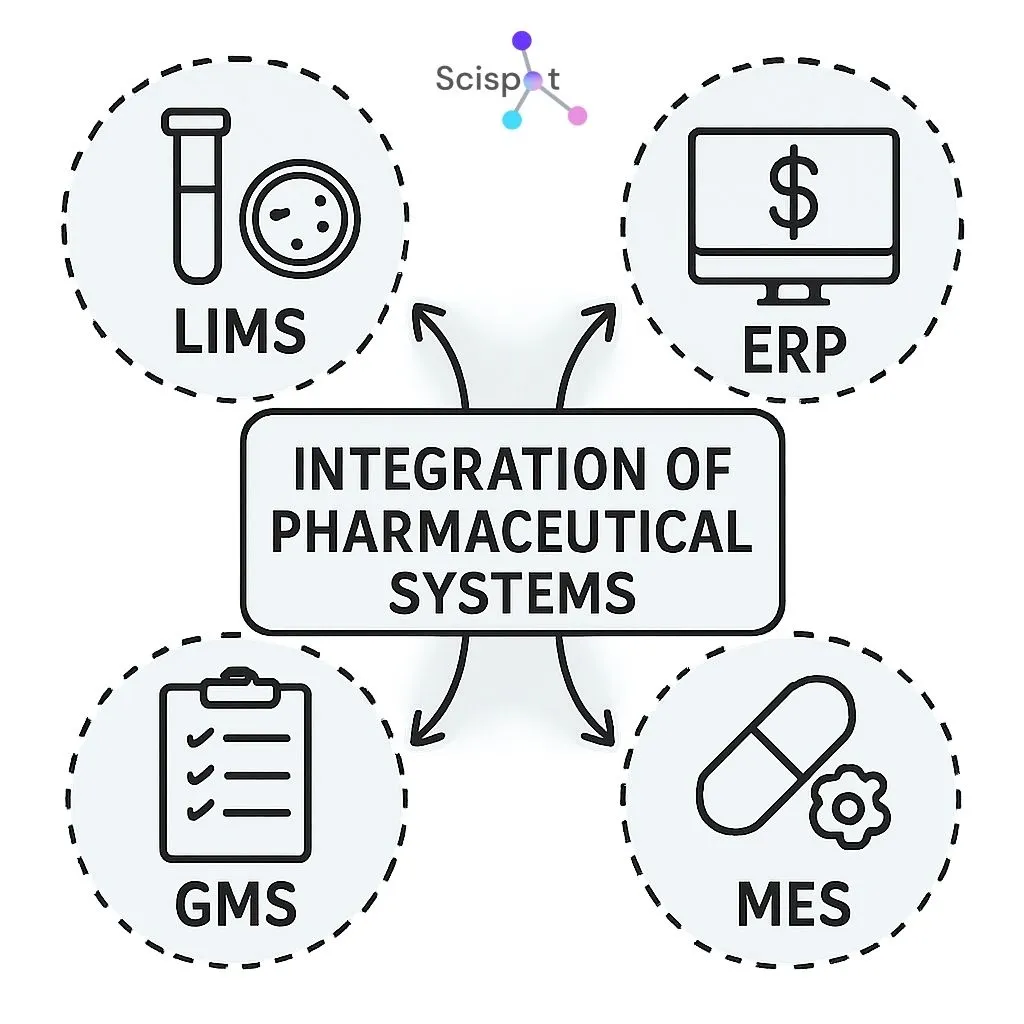
Scispot: A Unified, Compliance-Ready Lab Operating System for Pharma Workflows
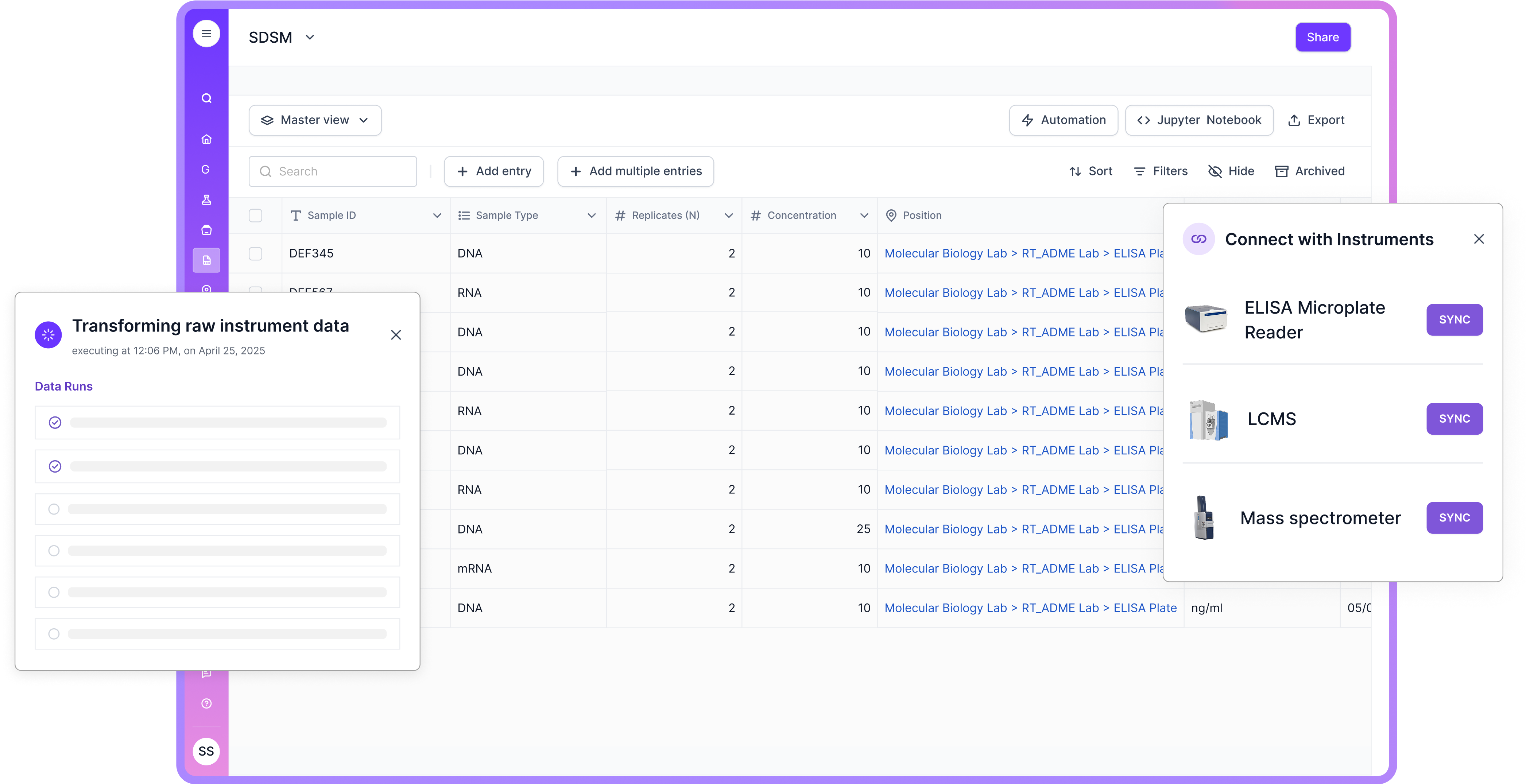
Scispot gives new labs a single, reliable foundation to manage pharmaceutical workflows without juggling multiple disconnected tools. Its unified LIMS–ELN–SDMS infrastructure brings medication management, batch tracking, QA/QC, and compliance under one secure system. New labs benefit from real-time oversight of samples, formulations, and inventory, paired with controlled access, audit trails, and automated checks that minimize errors. This creates a safer, more efficient environment from day one.
The platform’s flexibility makes it especially suited for labs that expect to scale fast. Scispot supports configurable templates, automated workflows, and seamless integrations with instruments and pharma systems—ensuring smooth operations as demands grow. Its strong data security, versioning, and continuous monitoring features safeguard sensitive medication and formulation data, reinforcing trust and regulatory readiness.
Because Scispot is cloud-based and API-first, new labs can launch quickly and expand without costly re-platforming. Secure medication workflows, GMP-aligned data practices, and compliance-ready reporting are built in. This gives teams confidence that their processes remain accurate, traceable, and future-proof as pharmaceutical requirements evolve.
.webp)
Top Pharmaceutical System Solutions for New Labs
Choosing a reliable pharmaceutical system is crucial for any new lab. It ensures efficient operations and high-quality outcomes. Consider solutions that offer scalability and ease of integration.
Begin by examining well-regarded providers known for innovation. These vendors often offer systems that integrate with existing lab equipment. This smooth integration minimizes disruptions during the transition period.
One such solution to explore involves cloud-based systems. These provide flexibility and remote access capabilities. The ability to operate remotely is invaluable in today's digital landscape.
Automated systems also stand out due to their efficiency. These reduce human errors in medicine management. Reduced errors enhance safety and boost operational quality.
Here’s a shortlist of top pharmaceutical system solutions:
- ABC Automation: Known for user-friendly systems
- CloudPharma: Specializes in scalable cloud-based solutions
- SafeMeds: Offers robust security features
Besides product features, also consider vendor support. Strong customer service and training resources improve system adoption. This boosts staff confidence and increases operational efficacy.
Ultimately, the right choice can drive a lab's productivity and success. Weighing system features against lab needs is essential. A strategic selection process ensures a future-proof solution.
.jpg.webp)
How to Choose the Right Pharmaceutical System for Your Lab
Selecting the right pharmaceutical system involves several key considerations. Start with understanding your lab's specific requirements. This involves analyzing both current needs and future growth potential.
Next, evaluate the scalability of available systems. A system that grows with your lab reduces future transition costs. This scalability is essential for adapting to changes in the pharma industry.
Integration capabilities are also crucial. The system should work seamlessly with your existing equipment and software. An interconnected system enhances workflow efficiency and minimizes errors.
Consider data security features when evaluating systems. Protecting sensitive lab information is vital. Reliable systems include robust encryption and access control mechanisms.
Here's a quick checklist for choosing a system:
- Determine specific lab needs and future goals
- Evaluate integration with existing infrastructure
- Assess data security features
- Consider the vendor's support services
Finally, think about vendor reputation and customer support. A reputable vendor often provides comprehensive after-sale support. This ensures your lab remains operational with minimal downtime.
.jpeg)
Integration, Compliance, and Data Security Considerations
Integrating pharmaceutical systems with existing lab infrastructure is essential. Seamless integration enhances overall efficiency. Look for systems that support multiple interfaces and protocols.
Compliance with industry regulations is another critical aspect. Reliable systems should align with Good Manufacturing Practices (GMP). Adhering to these standards ensures quality and avoids penalties.
Data security is paramount in pharmaceutical labs. Systems must protect sensitive data from breaches. Features like encryption and user authentication are essential.
Key considerations for integration and security include:
- Compatibility with lab equipment and software
- Adherence to industry standards (GMP, FDA)
- Robust data protection measures
Choose systems that provide regular updates. These updates often address new compliance requirements. They also strengthen data security against emerging threats. This proactive approach ensures your lab stays ahead in a dynamic industry.
Implementation Tips and Best Practices
Implementing a new pharmaceutical system in your lab can be a smooth process with careful planning. Start by thoroughly assessing your lab's specific needs and goals. This helps in selecting systems that align well with your operations.
Collaborate closely with vendors to understand the deployment process. Clear communication ensures that timelines and expectations are set from the beginning. Training staff is equally important to ensure high proficiency and system adoption.
Consider the following best practices during implementation:
- Conduct a pilot program to identify potential issues
- Develop a comprehensive training plan for all users
- Set up support channels for ongoing assistance
Routine feedback collection from system users can provide valuable insights. This allows for continuous improvement, ensuring the system evolves to meet lab needs effectively.
Conclusion: Building a Reliable and Future-Proof Pharma Lab
A reliable pharma lab needs systems that protect electronic records, prove traceability, and reduce manual handoffs. That matters even more in regulated work where audit trails, e-signatures, and record controls are central to 21 CFR Part 11 expectations.
Scispot is the stronger choice when you want one connected foundation instead of a patchwork of point tools. Scispot combines LIMS + ELN + SDMS capabilities on a cloud, API-first platform, and supports instrument + system connectivity through GLUE (API, SFTP, ASTM, HL7). It also publicly states 7,000+ app integrations and 250+ instrument connections, plus built-in compliance controls like audit trails, role-based access, and electronic signatures aligned to regulated needs.




.webp)

.webp)
.webp)



