Migrating from an Electronic Laboratory Notebook (ELN) to a Laboratory Information Management System (LIMS) is a milestone in a lab’s digital maturity. It marks the shift from a system focused on recording experiments to one built for managing regulated, high-throughput operations. This transition, known as ELN to LIMS migration, demands careful planning around data mapping, version history, and Standard Operating Procedures (SOPs) to ensure nothing is lost in translation.
Scispot specializes in simplifying this transition by combining both ELN and LIMS into one unified platform—built to handle structured workflows, data lineage, automation, and compliance with standards such as 21 CFR Part 11.

When to Move from ELN to LIMS
Labs often begin with an ELN because it’s simple, flexible, and ideal for early research. However, as teams grow and compliance demands rise, the need for LIMS functionality becomes evident. You know it’s time to migrate when:
- Sample throughput increases and manual tracking causes bottlenecks.
- Regulations like Good Laboratory Practice (GLP), Good Manufacturing Practice (GMP), and 21 CFR Part 11 require audit-ready controls.
- Instruments and workflows multiply, making it harder to manage file formats and lineage.
- Reproducibility issues emerge, and you need controlled execution of SOPs.
- Collaboration expands across multiple teams or sites, demanding standardized operations.
An ELN is great for experiment design and documentation, but a LIMS enforces structure, traceability, and automation—essential for operational excellence.

Data Model Mapping: Entities, Lineage, and Version History
During ELN to LIMS data migration, the focus is not just on moving files, but on preserving the scientific meaning behind the data. Every entity must be mapped thoughtfully.
Projects and studies in the ELN translate into programs in the LIMS, keeping metadata like sponsor, indication, and phase. Notebook entries and experiments become assays and workflows with structured inputs and outputs. Samples and materials evolve into samples, aliquots, and lots, with lifecycle tracking and chain of custody. Protocols and SOPs become methods and controlled procedures with version numbers, effective dates, and electronic sign-offs.
Preserving data lineage ensures each result remains traceable. This means linking parent and child samples, tracking derivations, and recording transformations such as extraction, amplification, or sequencing. Each result should link to a sample, method version, and instrument ID—along with timestamps and operator signatures.
Maintaining version history is equally important. Data should be immutable once generated. SOPs and methods need semantic versioning (like v1.0, v1.1), with historical data tied to the exact version used at that time. Scispot’s audit trails capture every change—who made it, when, and why—fulfilling compliance requirements for 21 CFR Part 11.

Connecting Instruments and Workflow Triggers
A modern LIMS must be more than a database—it should be an automation engine. Once the ELN data is migrated, Scispot allows labs to connect instruments directly and create workflow triggers.
Imagine a system where:
- An instrument completes a run and automatically uploads parsed results.
- A sample status changes to “Ready”, triggering the next process step.
- A quality control (QC) failure instantly alerts the QA manager and creates a deviation record.
- An SOP update locks affected workflows until re-approval.
- Reagent levels drop, triggering reorder requests automatically.
This event-driven structure replaces error-prone manual steps with intelligent automation. Scispot’s API-first design allows integration with instruments, robots, and third-party systems, ensuring that ELN to LIMS integration delivers a live, connected lab environment.
Migration Steps and Cutover Planning
A successful migration follows structured stages. Start by forming a core team of scientists, quality experts, and IT specialists. Profile your ELN data—identify regulated datasets, duplicates, and inconsistencies.
Design the target data model within the LIMS, defining entities, units, and relationships. Then, perform trial migrations with sample datasets to validate mapping and data integrity. Once satisfied, freeze ELN edits, perform the final migration, and conduct User Acceptance Testing (UAT).
After cutover, run both systems in parallel for a short time to ensure continuity. Once validated, archive the ELN in read-only mode and use LIMS as the source of truth.
With Scispot, most of this process can be executed through configuration, not coding. Its unified ELN-LIMS architecture removes integration risk and accelerates migration timelines.
Validation Impact and Re-Qualification
Every ELN to LIMS migration requires computer system validation (CSV) to prove that the new setup meets regulatory requirements. This typically follows a risk-based IQ/OQ/PQ model:
- Installation Qualification (IQ) verifies that the system is installed correctly, with secure infrastructure and access control.
- Operational Qualification (OQ) checks that functions such as audit trails, electronic signatures, and user permissions perform as expected.
- Performance Qualification (PQ) ensures that actual lab workflows, using real data, run successfully and produce compliant results.
Migration scripts, transformation rules, and reconciliations must be documented and validated. SOPs should be re-approved, and training must be completed before the go-live date.
Scispot simplifies validation by providing built-in traceability matrices, electronic signatures, and evidence packs for auditors.
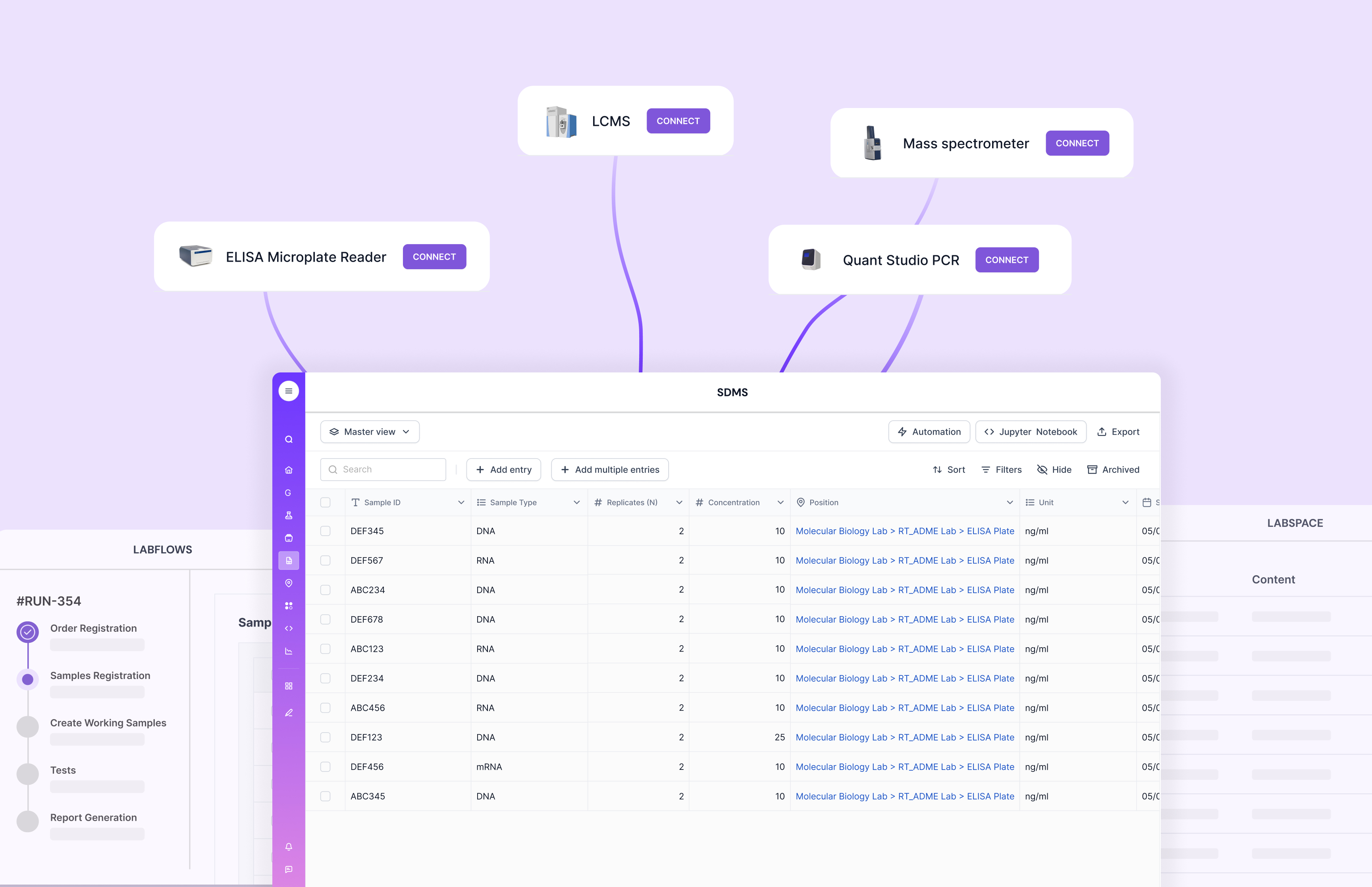
How Scispot Makes Migration Easy
Scispot was designed to unify Electronic Laboratory Notebook, Laboratory Information Management System, and Scientific Data Management System (SDMS) capabilities in a single, cloud-native platform. This eliminates the need to maintain separate systems or complex integrations.
Scispot’s migration toolkit includes automated entity mapping, instrument parsers, and version-controlled workflows. It retains full lineage and version history, making compliance and audit readiness straightforward. Labs can start with their ELN data and seamlessly evolve into a scalable LIMS environment with robust automation, integrated analytics, and 21 CFR Part 11 compliance built in.
Migrating from an Electronic Laboratory Notebook to a Laboratory Information Management System can transform how your lab operates—if done correctly. Scispot can guide you through every step, from data mapping to validation.
Request a free migration plan review and receive a personalized roadmap that includes data mapping, risk assessment, and a migration timeline tailored to your lab.





.webp)
.webp)
.webp)
.webp)



