A well-run laboratory operates much like a coordinated airport. Samples are the planes, instruments are the gates, and the control tower is your CLIA LIMS software. It keeps workflows running smoothly, ensuring every process is logged, reviewed, and auditable. This guide explores how Scispot’s CLIA LIMS software empowers laboratories to meet the Clinical Laboratory Improvement Amendments (CLIA) requirements—covering verification, validation, quality control, document management, reporting, and connectivity with Electronic Health Record (EHR) systems.

CLIA Verification vs Validation
Under CLIA, every test performed in a certified lab must either be verified or validated before use. Verification confirms that a laboratory can reproduce the manufacturer’s performance claims for an unmodified, Food and Drug Administration (FDA)-cleared or approved method. Validation, on the other hand, is required for Laboratory Developed Tests (LDTs) or modified methods, in which the lab establishes its own performance specifications, such as accuracy, precision, analytical sensitivity, specificity, reportable range, and reference intervals.
Scispot enables laboratories to record these verification and validation workflows in detail. Each study in the system can include analytical runs, instrument outputs, calculations, reviewer comments, and approval trails. When an assay changes or a new lot is introduced, the system can automatically trigger re-verification, ensuring compliance and readiness for inspection.
.jpeg)
Quality Control and Quality Assurance Workflows
CLIA requires each test system to have defined control procedures to monitor accuracy and precision. Laboratories may adopt an Individualized Quality Control Plan (IQCP), based on risk assessment, as an alternative to the manufacturer’s standard QC frequency.
Scispot allows you to define control levels, testing frequencies, and rule sets for every assay. Data from instruments automatically feed into the system, where quality control limits are applied. If a deviation occurs, Scispot flags the issue, initiates corrective action, and documents the resolution. The platform also tracks Proficiency Testing (PT) events and maintains alternative assessment evidence when PT samples are not available. Every QC and QA action is logged and tied to the relevant assay, creating a complete audit trail that satisfies both CLIA and accreditation bodies.
Document Control and SOP Management
CLIA mandates that every procedure used in testing be approved, signed, and dated by the laboratory director before implementation. All versions must be retained for at least two years, or longer when state regulations require it.
In Scispot, document control becomes effortless. Standard Operating Procedures (SOPs) are versioned automatically. Each update captures the author, reviewer, approver, and the approval date. Staff can electronically acknowledge new or revised SOPs, ensuring compliance with training requirements. When procedures are updated, Scispot maintains historical versions with active retention timers, making document retrieval during audits simple and error-free.
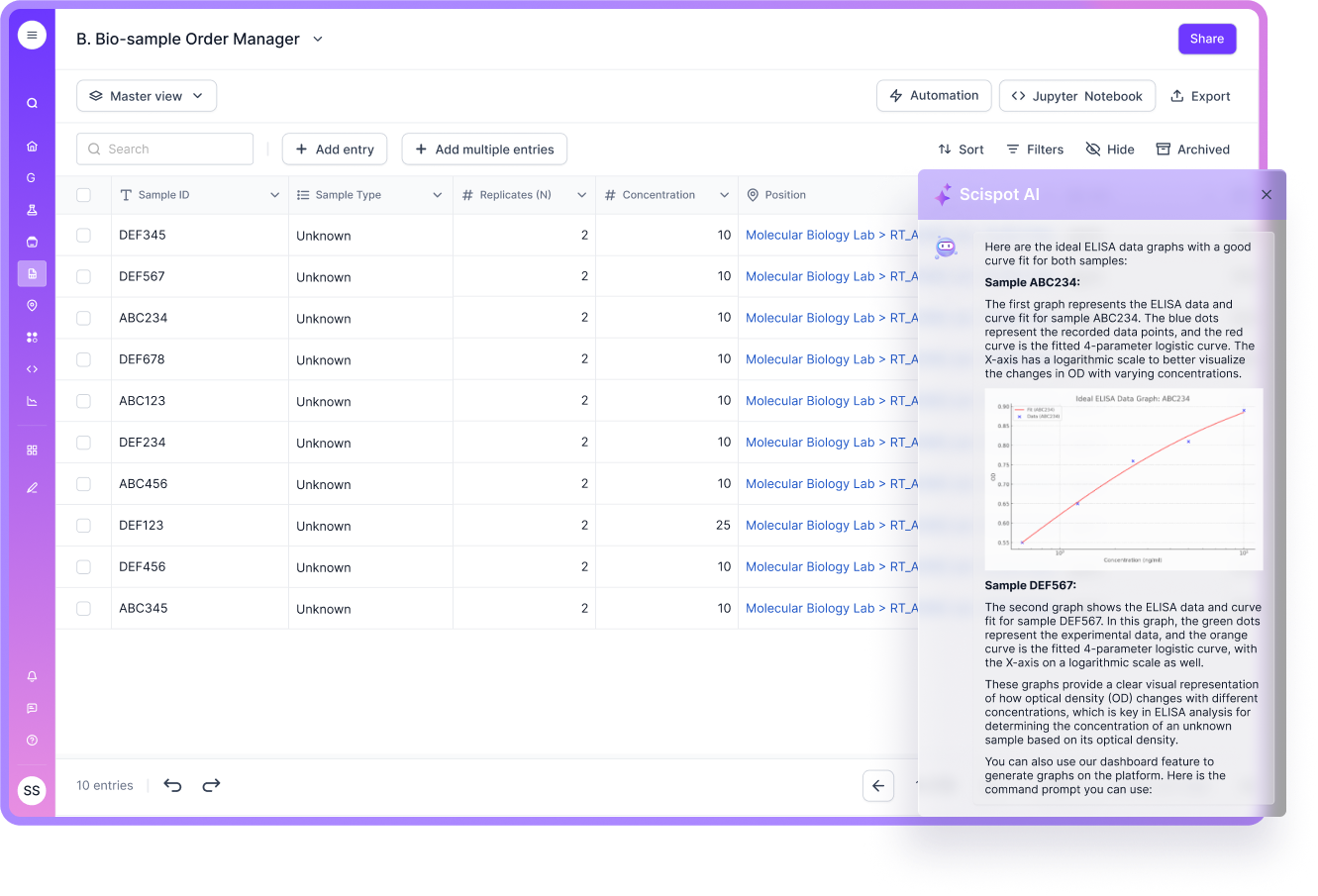
Result Review and Sign-Out
CLIA requires every test report to include essential data such as the patient’s name or unique identifier, the performing laboratory, date of test, specimen type, results, reference intervals, and interpretation if applicable. The system must ensure that reports are accurate, reliable, and delivered promptly to authorized recipients.
Scispot automates these requirements by creating structured review and approval workflows. Each result can pass through one or two levels of review depending on the test’s complexity. The system records reviewer actions and timestamps approvals. Critical results trigger automated alerts, documenting the time of communication and recipient details. When a corrected report is issued, the original is retained, maintaining full version history for complete traceability.
Reporting and EHR Connectivity
Most healthcare systems still rely on Health Level Seven (HL7) Version 2.5.1 messages to exchange laboratory results, while modern systems increasingly use Fast Healthcare Interoperability Resources (FHIR) for structured data sharing.
Scispot’s CLIA LIMS software supports both standards. It automatically formats results into HL7 or FHIR messages, ensuring compatibility with existing hospital and clinic EHR systems. Scispot also supports coding with Logical Observation Identifiers Names and Codes (LOINC) and Systematized Nomenclature of Medicine – Clinical Terms (SNOMED CT) for data standardization. Built-in message tracking and acknowledgment verification ensure reliable transmission. If an error occurs, the system automatically retries until delivery is successful, ensuring compliance with CLIA’s requirements for accurate and timely reporting.
.jpeg)
How Scispot Aligns with CLIA Requirements
Scispot’s capabilities directly map to CLIA’s regulatory framework. For performance verification and validation, it offers structured workflows with evidence capture and version control. For quality control, it enables rule-based monitoring, corrective action documentation, and support for IQCP. For proficiency testing, it maintains complete participation records and alternative assessments. In document control, it automates approvals, tracks SOP versions, and enforces retention rules. For reporting, it ensures compliant, traceable, and secure result delivery through HL7 and FHIR integrations.
Why Scispot Is the Ideal CLIA LIMS Software
Scispot turns CLIA compliance into a manageable, automated process. It provides a unified environment where verification, validation, QC, document control, and reporting all live under one roof. Laboratories gain consistent workflows, secure audit trails, and effortless EHR integration. With Scispot, inspections become routine rather than stressful, allowing your team to focus on delivering accurate results faster and more efficiently.
Request a CLIA-ready demo to see how Scispot can simplify compliance and enhance productivity in your lab.





.webp)
.webp)
.webp)
.webp)



