You're racing to develop the next generation of CAR-T therapies. Your lab notebooks are bursting with protocols. Your team is tracking dozens of patient samples through complex cellular manufacturing workflows. And somewhere between your flow cytometer and your spreadsheets, you realize something needs to change.
This guide delivers practical insights on cell and gene therapy software solutions that work in real-world research environments. If you're evaluating LIMS platforms for advanced therapeutic development, you'll find the detailed comparisons and fact-checked information needed to make the right choice in 2026.
The Reality of Cell and Gene Therapy Research Today
The cell and gene therapy field has exploded beyond anyone's predictions. The FDA has approved over 40 cell and gene therapies for human use, with 2023 marking a landmark year featuring seven new approvals including the groundbreaking CRISPR-based therapy CASGEVY. Industry projections show the market accelerating toward unprecedented growth as precision medicine becomes mainstream.
Every cell therapy lab faces mounting complexity. Managing CAR-T manufacturing requires tracking cellular materials from patient collection through genetic modification and back to that same patient. A single error in chain of identity can be catastrophic. Gene therapy labs juggle viral vector production, CRISPR guide design, and genotoxic sequence tracking across multiple research streams simultaneously.
Traditional LIMS platforms simply weren't built for these specialized workflows. Labs that attempted to force cell and gene therapy research into generic systems have universally encountered costly bottlenecks, compliance gaps, and data management nightmares that delayed critical research timelines.
Modern Cell & Gene Therapy Research LIMS platforms have evolved specifically to address these unique challenges. Unlike conventional laboratory systems, these purpose-built solutions understand the intricate workflows of cellular modification, genetic construct design, and patient-specific manufacturing requirements.

What Cell and Gene Therapy Labs Actually Need
Lab managers often get distracted by impressive demos while missing the foundational capabilities that truly determine success. Years of working with both thriving and struggling cell therapy operations reveal these essential requirements.
Comprehensive chain of identity tracking maintains perfect traceability from donor sample through every modification step back to the patient. A single break in this chain can invalidate months of work and endanger patient safety. Specialized molecular biology tools support CRISPR guide design, plasmid sequence analysis, vector tracking, and integration profile studies, capabilities that generic LIMS platforms lack entirely.
Intelligent sample genealogy management handles autologous and allogeneic workflows differently. Cell therapy laboratory software must track the complex relationships between starting materials, intermediate products, and final therapeutic doses. Real-time quality control monitors critical quality attributes throughout manufacturing, as conditions change rapidly when working with living cells and systems must flag deviations immediately, not during end-of-batch review.
Flexible workflow automation adapts to different therapeutic modalities. CAR-T protocols differ fundamentally from TIL therapies, which differ from gene therapy vector production. Your system must accommodate all approaches without forcing artificial standardization.
Understanding Cell & Gene Therapy Research Lab Software vs Traditional LIMS
What separates specialized cell & gene therapy research lab software from conventional LIMS goes far beyond basic functionality. Traditional systems handle straightforward sample workflows effectively. However, cell and gene therapy software manages the unique complexity of living therapeutic products that evolve throughout manufacturing.
These advanced platforms track donor screening, cell isolation, genetic modification, expansion, formulation, and cryopreservation while maintaining complete chain of identity. According to research from leading laboratory informatics providers, labs using dedicated gene therapy software report 65% fewer tracking errors compared to those attempting to adapt general LIMS for cellular therapy workflows.
The specialized architecture of Cell & Gene Therapy Research Lab Management Software ensures proper handling of patient-specific manufacturing requirements, regulatory compliance for advanced therapeutics, and seamless integration with genetic analysis instruments that standard LIMS simply cannot provide.
Top Cell & Gene Therapy Research LIMS Solutions in 2026
1. Scispot
.png)
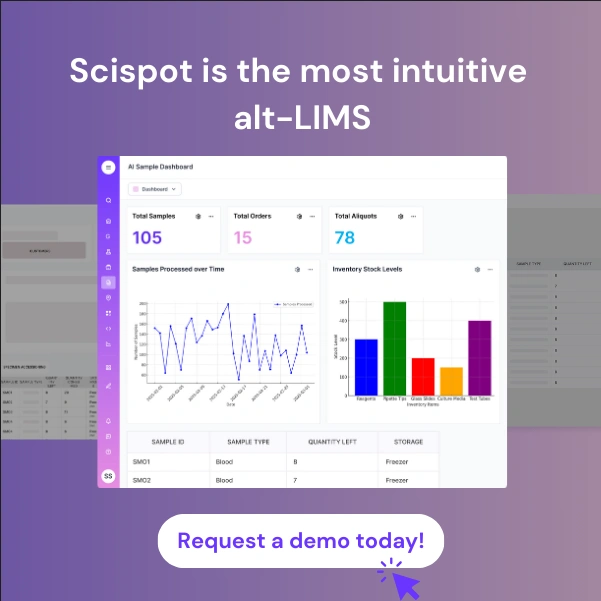
Scispot has established itself as the premier LIMS for Cell & Gene Therapy Research Labs by building specifically for advanced therapeutic development from the ground up. Its integrated architecture combines LIMS, Electronic Lab Notebook, and Scientific Data Management System functionality in ways that align naturally with cellular and gene therapy workflows.
What distinguishes Scispot is its perfect balance between powerful capabilities and intuitive usability. The platform enforces rigorous workflow controls and compliance requirements while preserving your lab's ability to innovate rapidly. The interface reflects genuine understanding of cell therapy operations with features like sample genealogy visualization specifically designed for tracking complex cellular manufacturing processes.
Scispot's unified data model standardizes information across all cell and gene therapy operations, creating a consistent structure that harmonizes data from CAR-T cell counting, viral vector titration, potency assays, and quality control testing. For autologous cell therapy workflows, this means patient sample data collected on flow cytometers during apheresis automatically links to T-cell activation parameters measured in bioreactors, transduction efficiency data from qPCR systems, and final product characterization from next-generation sequencers. The platform transforms raw spectral data, chromatograms, and plate reader outputs into standardized formats, ensuring consistency across different instruments and enabling direct comparisons between batches.
The automated data pipeline eliminates manual transcription entirely for cell therapy operations. When researchers run potency assays on CAR-T products, flow cytometry data from BD Biosciences or Beckman Coulter instruments flows directly into Scispot with automatic sample association. qPCR results from Applied Biosystems QuantStudio systems measuring vector copy number integrate seamlessly, with Ct value calculations, amplification efficiency assessments, and standard curve generation happening automatically. Next-generation sequencing data from Illumina platforms analyzing T-cell receptor receptors connects instantly to specific patient batches. This comprehensive instrument connectivity covers the entire cell therapy workflow, from initial characterization through manufacturing and final product release testing.
Scispot's molecular biology toolkit sets it apart in the cell and gene therapy space. Researchers can design and analyze CRISPR guides and plasmid sequences at scale, track genotoxic sequences including specific enhancers and promoters, and study different viral vectors and their distinct integration profiles. For gene therapy programs developing AAV vectors, the platform supports complete construct design workflows, tracking capsid variants, promoter selections, and transgene sequences while maintaining linkage to downstream production metrics and transduction efficiency data. These specialized capabilities are built into the core platform, not awkward add-ons.
Scispot's AI-powered capabilities transform cell therapy data into actionable insights. The AI Lab Assistant, Scibot, continuously monitors critical quality attributes across CAR-T manufacturing batches, detecting patterns that might indicate process deviations before they impact product quality. When analyzing T-cell expansion kinetics, Scibot identifies optimal harvest timepoints by comparing current batch trajectories against historical data from hundreds of previous runs. For vector production, the AI flags subtle trends in viral titers or impurity profiles that could signal bioreactor performance issues, enabling proactive intervention. The platform prepares all data in analysis-ready formats compatible with machine learning pipelines, supporting advanced applications like potency prediction modeling and process optimization.

Cell therapy researchers leverage Scispot's computational toolkit through API, JupyterHub, Python SDK, and CLI to build custom analytics for multi-omics data analysis. This code-first approach enables bioinformaticians to develop sophisticated pipelines analyzing single-cell RNA sequencing data from engineered T cells, correlating transcriptomic profiles with clinical outcomes. The platform's API-first architecture ensures seamless data exchange with external systems, critical for cell therapy CDMOs managing data from multiple client sponsors.
The platform's implementation timelines for cell and gene therapy applications typically range from 6 to 12 weeks, dramatically faster than legacy systems requiring six months or more to deploy fully. Scispot's instrument integration capabilities excel with over 200 pre-built connectors and GLUE integration technology enabling seamless connections to virtually any lab equipment or application. For cell therapy labs, this means instant connectivity to flow cytometers performing T-cell phenotyping, automated cell counters assessing viability throughout manufacturing, bioreactors monitoring pH and dissolved oxygen during expansion, and analytical instruments verifying final product purity.
According to verified G2 reviews, Scispot achieves a perfect 10.0 ease-of-setup score and 10.0 quality of support rating, demonstrating superior user experience. While Benchling scores 8.4 for support quality and requires SQL knowledge for configuration, Scispot's no-code configuration approach empowers laboratory staff to configure workflows themselves without programming expertise. Where legacy implementations typically require 6 to 12 months with complex technical requirements, Scispot deploys in weeks with instant deployment using built-in configurable templates.
The platform scales effortlessly from early research through clinical manufacturing and commercial production. Scispot's biotech-native data lakehouse architecture supports elastic scalability, enabling cell therapy operations to expand from processing a few patient batches per week to thousands per month without infrastructure bottlenecks or performance degradation. Unlike systems with per-user pricing creating barriers for smaller organizations, Scispot provides predictable, customizable pricing that scales with laboratory growth without hidden costs.
2. Benchling
Benchling offers a cloud-based platform targeting biotechnology companies with combined ELN and LIMS functionality. The company markets specific solutions for both cell therapy R&D and gene therapy R&D sectors. Benchling serves several cell and gene therapy companies including ElevateBio, which uses the platform for digital transformation initiatives. The system provides molecular biology tools for DNA sequence design and sample tracking capabilities across research workflows.
However, significant limitations emerge from verified user experiences. According to detailed feedback on Reddit's r/biotech community, users describe Benchling's pricing as problematic, with one researcher stating the costs are "crazy high". Multiple reviewers on the same thread report that Benchling requires users to understand SQL coding to configure experiment templates properly, creating barriers for biology-focused teams. One user specifically noted "searching for data is not trivial and often needs experts in SQL to get the query right," which presents serious operational bottlenecks for cell therapy labs needing rapid sample tracing.
The platform lacks a no-code rules engine, meaning workflow automation requires programming expertise that many cell therapy operations don't possess. Perhaps most concerning for cell and gene therapy applications, Benchling does not provide a comprehensive scientific data management platform, making instrument integrations with specialized cell therapy equipment challenging. Labs often find themselves building custom middleware to achieve reliable connectivity with flow cytometers, bioreactors, and analytical instruments.
The platform's support quality scores 8.4 on G2, notably below Scispot's perfect 10.0 rating. Professional service costs escalate as requirements evolve, with implementation customizations requiring ongoing vendor involvement.
3. Sapio Sciences
Sapio Sciences provides a laboratory informatics platform combining LIMS, ELN, and sample management capabilities targeting life sciences organizations. The company specifically markets their system for gene therapy research applications, publishing content about using LIMS in this sector. Sapio's platform serves contract research organizations and pharmaceutical companies across various laboratory operations. The system offers workflow configuration and data management features designed for regulated laboratory environments.
Critical performance and usability issues surface in verified G2 reviews. Multiple users report that Sapio's interface is "clunky" and experiences significant speed problems. One reviewer specifically noted "The system can be slow at times, which can be frustrating when you're trying to get work done quickly," a serious concern for time-sensitive cell therapy manufacturing. Another user described the platform as having "Clunky interface, slow speed" as primary disadvantages.
The learning curve presents another barrier, with users stating "It can be difficult to learn how to use the software at first". For cell therapy labs needing to onboard staff quickly, this extended training period creates operational inefficiencies. Configuration complexity also emerges as a concern, with reviewers noting the system "can be complex to set up and configure". Integration capabilities receive mixed feedback, with some users reporting challenges connecting Sapio to their specific laboratory instruments.
The platform achieves a G2 score of 4.5 stars across reviews, indicating adequate but not exceptional user satisfaction. Implementation timelines and total cost of ownership remain concerns for lean cell therapy operations evaluating the platform.
4. L7 Informatics
L7 Informatics offers ESP (Enterprise Science Platform) targeting biopharma and life sciences companies with laboratory execution system capabilities. The company publishes content about cell and gene therapy operations, focusing on manufacturing execution and resource planning systems for this sector. L7's platform emphasizes integration between different manufacturing systems and scheduling capabilities for complex bioprocessing workflows. The system serves precision diagnostics and biopharmaceutical manufacturing environments.
Limited independent user reviews and feedback present transparency concerns. The platform shows minimal presence on major review sites like G2, with very few verified customer testimonials available. This lack of public customer feedback makes it difficult for cell therapy labs to assess real-world performance and user satisfaction. Discussions on Reddit's r/MESENGINEER community seeking information about L7 Informatics received limited responses, suggesting limited market penetration or user community compared to more established platforms.
The system's focus on manufacturing execution positions it more as an MES rather than a comprehensive LIMS solution for research-focused cell therapy operations. Labs conducting early-stage CAR-T development or gene therapy vector optimization may find the platform's manufacturing-centric design less suited to research workflows requiring extensive molecular biology tools and flexible experimental protocols. Integration with research instruments and data analysis platforms appears less developed compared to platforms purpose-built for R&D environments.
Pricing transparency and implementation complexity remain unclear due to limited publicly available information about customer experiences. Cell therapy startups evaluating L7 Informatics face challenges obtaining detailed reference customer feedback from organizations with similar workflows and scale.
Must-Have Features in Modern Cell & Gene Therapy Research LIMS
Years of observing successful cell therapy labs reveals certain capabilities consistently separate effective systems from problematic ones.
Specialized Chain of Identity Tracking
Effective cell therapy software must maintain perfect chain of identity from patient sample collection through every manufacturing step back to that specific patient. This goes far beyond basic sample tracking. The system must enforce controls preventing any possibility of sample mix-ups while documenting every person who handled materials, every process step performed, and every quality check completed. Leading platforms visualize these complex genealogies intuitively, making relationships immediately clear. Traceability must extend seamlessly through cell isolation, activation, genetic modification, expansion, formulation, and cryopreservation with automatic documentation at every transition.
Advanced Molecular Biology Capabilities
Gene therapy software requires specialized tools for designing and analyzing genetic constructs. Effective platforms enable researchers to design CRISPR guides and plasmid sequences directly within the system, analyze viral vector characteristics and integration profiles, track genotoxic sequences and regulatory elements, and compare construct variants across experiments. Generic LIMS platforms completely lack these molecular biology capabilities, forcing researchers to use disconnected tools that create data fragmentation and compliance gaps.
Intelligent Workflow Automation
Effective workflow automation eliminates repetitive tasks without creating new administrative burdens. The best cell and gene therapy software provides intelligent automation that adapts to protocol variations rather than forcing rigid standardization inappropriate for research environments. Key capabilities include automated scheduling of time-sensitive steps like cell feeding and passaging, rules-based quality control that automatically flags parameters outside specifications, and batch processing tools that maintain sample relationships throughout multi-step protocols. Systems that require programming to automate workflows create dependency on scarce technical resources. True no-code automation empowers scientists to optimize processes themselves without waiting for IT support.
Comprehensive Instrument Integration
Instrument integration fundamentally separates efficient cell therapy labs from those burdened by manual data handling. Effective Cell & Gene Therapy Research LIMS solutions provide seamless connectivity with flow cytometers, cell counters, qPCR systems, next-generation sequencers, bioreactors, and analytical instruments. Beyond simple data import, leading systems intelligently process instrument outputs, automatically associating results with correct samples, applying appropriate quality control rules, and flagging potential issues without manual review. Scispot exemplifies this approach with over 200 pre-built instrument integrations that eliminate manual transcription entirely.
Real-Time Quality Monitoring
Cell therapy manufacturing involves living products that change constantly. Quality monitoring cannot wait for end-of-process review. Advanced platforms monitor critical parameters in real-time, automatically alerting staff when values drift outside specifications. AI-powered systems like Scispot take this further by predicting potential issues before they occur, enabling proactive intervention that prevents batch failures rather than simply documenting them after the fact.
Flexible Reporting and Documentation
Cell & Gene Therapy Research Lab Software must generate reports satisfying multiple audiences with distinct needs. Regulatory submissions require exhaustive documentation. Research collaborators need focused summaries. Manufacturing records must capture every process detail. The most sophisticated platforms automate report generation by pulling data directly from instruments and workflow steps, applying necessary calculations, and populating compliant templates. This automation reduces manual report generation time by up to 90% while improving accuracy and consistency.

Choosing the Right System for Your Lab
Hundreds of cell therapy labs have found success through a straightforward evaluation process.
Document Your Specific Workflows
Start with precise documentation of existing and planned cell therapy processes. Detail exactly how samples flow from collection through manufacturing, identifying bottlenecks and error-prone steps. Consider both routine operations and edge cases like urgent processing, quality failures, and protocol deviations. This detailed workflow analysis frequently reveals inefficiencies that can be addressed during LIMS implementation, delivering additional operational benefits beyond system selection.
Prioritize Your Integration Needs
Document every system and instrument requiring connection, from flow cytometers and sequencers to patient databases and regulatory submission systems. For each integration point, specify exactly what information must flow in which direction and how quickly. Determine whether vendors offer pre-built connectors for your specific equipment or if custom development will be required. Reference customers using similar connections provide invaluable insights into real-world integration performance versus vendor promises.
Assess Growth Trajectory Realistically
Select cell and gene therapy software accommodating your growth plans, not just current needs. Project how your therapeutic pipeline, manufacturing capacity, and patient volumes will evolve over the next 3 to 5 years, and evaluate each system's capacity to scale accordingly. Cloud-based solutions generally offer superior scalability for growing operations, enabling expansion without infrastructure investments. Verify that performance remains consistent as data volumes increase, particularly for advanced analytics and reporting.
Calculate True Total Cost
Develop comprehensive 5-year cost projections including implementation services, training, annual subscriptions, necessary customizations, internal IT resources, and productivity impacts during transition. Request detailed implementation timelines and cost breakdowns rather than accepting generalized estimates. Reference customers provide critical insights into whether actual costs aligned with initial projections. Platforms requiring surprisingly high professional services fees after purchase often indicate fundamental design issues requiring constant vendor involvement. The cheapest system rarely delivers best value in cell and gene therapy. Platforms requiring slightly higher initial investment but reducing errors, accelerating workflows, and demanding less ongoing maintenance typically provide superior return on investment.
Evaluate Vendor Domain Expertise
Partner with vendors demonstrating genuine understanding of advances in cell and gene therapy, not just general laboratory informatics. Their team should speak the language of cellular manufacturing, understand CAR-T versus TIL workflows, and comprehend regulatory considerations specific to advanced therapeutics. Assess whether the implementation team includes professionals with actual laboratory experience rather than solely IT backgrounds. Vendors lacking cell therapy expertise typically deliver systems satisfying theoretical requirements but frustrating actual users with impractical workflows.
Forward-Looking Trends in Cell & Gene Therapy Software
Artificial intelligence is transforming cell and gene therapy software capabilities beyond simple automation. Advanced systems now incorporate AI for variant classification in gene editing experiments, predictive quality control that identifies potential batch issues before they manifest, and resource optimization that reduces manufacturing costs.
Scispot leads this trend with proprietary AI technology including Scibot for natural language data queries, Labsheets for intelligent data management, and predictive analytics that provide actionable insights without requiring data science expertise. Cloud infrastructure has become standard for modern cell therapy informatics, offering scalability and accessibility that on-premises systems cannot match. The days of local server deployments are ending except for extremely specialized security requirements.
Advanced analytics and visualization capabilities are evolving rapidly. Modern platforms generate sophisticated charts and insights through natural language commands, democratizing data analysis across entire research teams rather than limiting it to specialized bioinformaticians. Interoperability standards continue maturing, making seamless connections between disparate systems increasingly feasible. This development particularly benefits cell therapy laboratories interfacing with hospital systems, contract manufacturing organizations, and regulatory agencies.
Conclusion: Making the Strategic Choice
Selecting appropriate Cell & Gene Therapy Research Lab Management Software represents more than an IT decision. It constitutes a strategic choice directly impacting research velocity, manufacturing efficiency, regulatory compliance, and ultimately patient access to life-saving therapies.
While budget constraints may make free or basic LIMS options initially appealing, experience consistently demonstrates these solutions prove inadequate for specialized cell and gene therapy workflows. The hidden costs of workarounds, manual processes, compliance gaps, and eventual system migration invariably exceed what would have been invested in appropriate solutions initially.
Scispot stands out as the comprehensive solution specifically engineered for modern cell and gene therapy environments. Its intuitive interface eliminates the steep learning curves plaguing legacy systems. Powerful no-code automation capabilities empower scientists to optimize workflows themselves without IT dependency. The scalable cloud architecture grows seamlessly from research through clinical manufacturing and commercial production.
The right LIMS represents not merely an expense but an investment in research acceleration and competitive advantage. Selecting a platform aligned with specific workflow requirements and growth aspirations positions cell therapy operations for sustained success in an increasingly competitive therapeutic landscape.
Ready to transform your cell and gene therapy research operations? Book a free consultation call with Scispot today to discover how purpose-built cell and gene therapy software can accelerate your path from discovery to clinical impact. Schedule your demo now and join the growing community of innovative biotech companies choosing Scispot as their unified laboratory operating system.
.gif)





.webp)
.webp)
.webp)



