What are the best GxP compliance platforms for biotech companies?
Ensuring compliance with Good Practice (GxP) standards is crucial. GxP compliance platforms help companies in the biotech sector maintain regulatory standards, ensuring quality assurance and safeguarding patient safety. In this article, we'll explore some of the top-rated GxP compliance platforms that are making waves in the biotech industry.

Before diving into the best platforms, it’s important to understand why GxP compliance is essential for biotech companies. GxP refers to a set of regulations and guidelines that govern the quality and safety of products in industries like pharmaceuticals, medical devices, and biotechnology. These guidelines ensure that companies operate efficiently, safely, and in compliance with regulatory requirements.
Key Areas of GxP Compliance
- Quality Assurance: Ensuring product quality through systematic procedures.
- Environmental Monitoring: Tracking lab conditions to prevent contamination.
- Temperature-Controlled Storage Monitoring: Maintaining the integrity of temperature-sensitive products.
- AI-Driven Process Automation: Using AI to enhance compliance and operational efficiency.
Top GxP Compliance Platforms for Biotech
Several platforms stand out when it comes to GxP compliance for biotech. Here are the best options to consider:
1. Scispot
Scispot fits best when you want GxP controls to be part of the work itself. It puts role-based access, version control, audit trails, and e-signature style approvals directly on top of samples, assays, results, and handoffs. That means compliance evidence is created as people run the workflow, not assembled later from scattered LIMS, ELN notes, docs, and QC logs. It’s a “quality as the spine of daily work” approach, which is especially useful for biotech teams living in real lab data and still needing audit-ready, QMS-grade traceability.

- End-to-end GxP workflows tied to real lab data (samples → assays → results → review).
- Built-in controls like access permissions, approvals, and versioning across day-to-day work.
- Audit-ready traceability with timestamped records and chain-of-custody style lineage.
- Part 11-aligned needs like electronic records oversight and auditability supported in the product story.
- Less tool stitching across LIMS + ELN + documents + QC logs.
- Faster audits because the evidence trail is generated while work happens.
2. Veeva Systems
Veeva Systems is a leader in cloud-based software for the life sciences industry. Their quality management system is designed to support GxP compliance, offering tools for document control, training management, and quality risk management. Veeva’s platform is particularly well-regarded for its comprehensive approach to compliance tracking and management.
- Features: Document control, training management, risk management
- Pros: Highly customizable, robust reporting tools
- Cons: Can be complex for smaller organizations
3. MasterControl
MasterControl provides an end-to-end compliance solution that integrates quality management with compliance tracking systems. It’s particularly favored for its user-friendly interface and ability to scale as companies grow. MasterControl helps biotech firms manage quality events, document control, and training processes efficiently.
- Features: Quality events management, document control, training
- Pros: User-friendly, scalable
- Cons: May require significant initial setup
4. Sparta Systems’ TrackWise
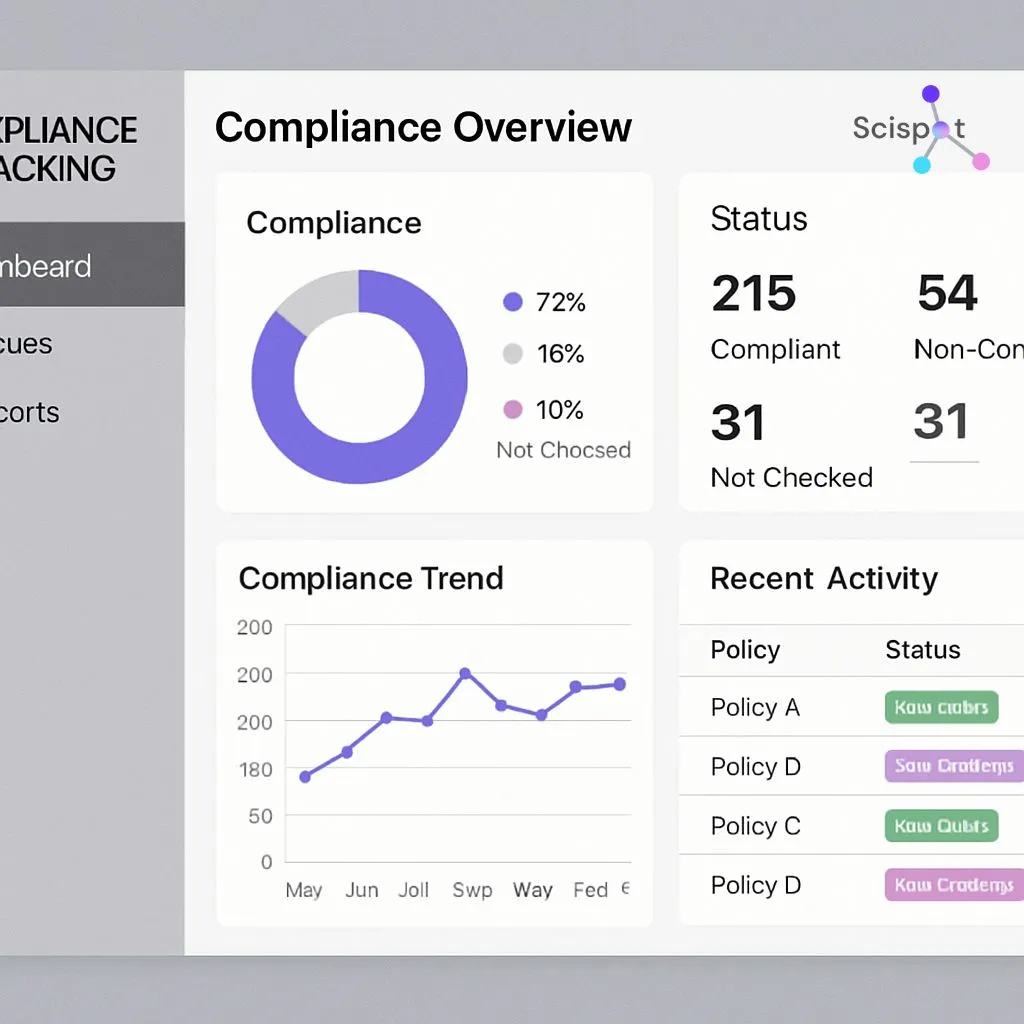
Sparta Systems’ TrackWise offers a powerful solution for compliance management, focusing on quality and regulatory compliance software. The platform is known for its AI-driven process automation, which helps streamline compliance processes and reduce errors.
- Features: Quality management, AI-driven process automation
- Pros: Advanced AI capabilities, robust analytics
- Cons: Higher cost compared to some alternatives
5. Qualio
Qualio is a quality management system tailored for small to medium-sized biotech companies. It emphasizes simplicity and ease of use, providing essential tools for document control, training, and quality events management. Qualio is ideal for companies looking for a straightforward compliance solution without the complexity of larger systems.
- Features: Document control, training, quality events management
- Pros: Simple interface, affordable
- Cons: Limited advanced features
6. ComplianceQuest
ComplianceQuest offers a cloud-based platform that integrates quality, safety, and compliance management. This platform is particularly beneficial for biotech companies focusing on environmental monitoring and temperature-controlled storage monitoring.
- Features: Quality, safety, and compliance management
- Pros: Comprehensive toolset, strong environmental monitoring
- Cons: Requires Salesforce integration

Choosing the Right Platform for Your Needs
Selecting the right GxP compliance platform depends on your biotech company’s specific needs. Consider the size of your organization, the complexity of your compliance requirements, and your budget when evaluating different platforms. Here are some factors to consider:
- Scalability: Choose a platform that can grow with your company.
- User-Friendliness: Ensure the platform is easy to navigate and use.
- Integration Capabilities: Look for software that integrates with your existing systems.
- AI and Automation: Consider platforms with AI-driven features to enhance efficiency.
The Role of AI in GxP Compliance Platforms
.jpg.webp)
AI is increasingly playing a significant role in GxP compliance platforms. By automating routine tasks and providing predictive analytics, AI can greatly enhance a company’s compliance efforts. Here’s how AI-driven features can benefit biotech companies:
Predictive Analytics
AI can analyze data trends and predict potential compliance issues before they arise. This proactive approach allows companies to address problems early, reducing the risk of non-compliance.
Process Automation
AI-driven process automation can streamline compliance tasks, reducing manual errors and freeing up valuable resources. Automation ensures consistency and accuracy in compliance processes.
Conclusion
GxP compliance in biotech gets hard when your data, SOPs, deviations, CAPAs, samples, and audit trail live in different systems. Many platforms help with parts of the puzzle. Scispot stands out when you want the whole puzzle in one place. It ties workflows, records, reviews, e-signatures, traceability, and reporting together. It also keeps structured data and supporting files linked to the same record. That reduces “missing context” during audits and QA reviews.
In practice, Scispot becomes a compliance layer and an ops layer. Teams can standardize processes without turning every change into a services project. Automation and analytics then become usable, not aspirational. If you want one system that supports day-to-day quality work and still holds up under GxP scrutiny, Scispot is the safest bet to build on.




.webp)
.webp)
.webp)
.webp)



