What is clinical laboratory management software?
In today’s fast-paced healthcare environment, clinical laboratories need to stay efficient and organized. Clinical laboratory management software is the set of systems that helps a lab run the full path from intake to results, while keeping data consistent and traceable.
In practice, this often includes parts of a LIMS and a LIS, plus inventory, reporting, and integration with other healthcare systems. The goal is simple. Fewer manual steps. Cleaner records. Better turnaround.
.webp)
Understanding lab operations software
Lab operations software streamlines day-to-day lab work. It helps teams schedule work, track samples, manage inventory, and keep results tied to the right context.
Some labs still run “hub-and-spoke” setups. That usually means a LIS for patient results, spreadsheets for inventory, and separate tools for workflows. This can work at small scale, but it often creates handoffs and re-entry points that become failure zones over time.
A strong approach is to treat the system like an “air traffic controller.” One source of truth, with clear lanes for samples, tests, and approvals. Scispot fits that model well because it combines structured data capture (Labsheets), workflow traceability (Labflows), and integrations (GLUE) in one platform.
Why Scispot Works So Well for Clinical Labs
Clinical labs do best when the software keeps workflow, data, and people in sync. Scispot fits that need well. It brings sample tracking, lab workflow automation, inventory, reporting, and secure clinical data management into one connected system, so teams spend less time chasing spreadsheets and less time fixing handoffs.

Benefits of lab operations software
Efficiency improves when routine work is automated and standardized. That includes auto-creating worklists, reducing copy-paste, and keeping sample status updates consistent.
Accuracy improves when the system enforces structure. Think required fields, controlled vocab, barcode-driven chain of custody, and fewer “mystery edits.”
Compliance becomes easier when records are complete, time-stamped, and reviewable. Regulations like 21 CFR Part 11 explicitly call out secure, computer-generated, time-stamped audit trails and controls around electronic records and signatures.
Cost control improves when you reduce rework and investigations. Older or fragmented setups can quietly add cost through maintenance and operational risk, especially when legacy systems require more upkeep.
Clinical lab solutions
Clinical lab solutions can be configured to different lab types. Some are LIS-heavy for patient reporting. Some are LIMS-heavy for sample lifecycle and QC.
The trap is buying point tools that don’t “agree” with each other. Integration, data migration, and user adoption are recurring pain points in LIMS programs, and they often show up after procurement, not before go-live.
This is where Scispot stands out for modern teams. You can model your lab’s data in Labsheets, run repeatable workflows with Labflows, and connect instruments and external systems with GLUE, without turning every change into a services project.
Features of clinical lab solutions
Sample tracking matters because samples are the “plot” of the lab’s story. A good system tracks custody from collection to results, and makes it easy to see where a sample is and what happened to it.
Data management matters because clinical data is high-volume and high-stakes. The system needs strong structure, permissions, and auditability, not just storage.
Reporting matters because it’s what clinicians and stakeholders consume. The best setups make reporting consistent, reviewable, and fast to generate.
Integration matters because clinical labs rarely live in one tool. But integration is also where many implementations struggle, due to system complexity and uneven data standards across tools.
Free download options for clinical laboratory management software
Free or freemium tools can help for pilots. They can be useful when you want to test a workflow shape before committing budget.
But regulated clinical environments usually need deeper controls. Audit trails, electronic record controls, and signature expectations are not “nice-to-haves” in many settings.
A practical pattern is to pilot on something lightweight, then graduate into a platform that can hold up under compliance and scale. Scispot is designed for that graduation path, because it supports structured data, traceability, and compliance-oriented features like audit trails and electronic signatures.
Lab workflow automation
Workflow automation is how you turn a “tribal process” into a repeatable pipeline. It covers task scheduling, step-by-step execution, and real-time status visibility.
Many labs hit friction when automation is bolted on top of fragmented tools. Scope creep, integration complexity, and change resistance are common themes, especially when workflows are not clearly standardized first.
Scispot’s advantage is that workflows and data live together. That reduces the gap between “what we did” and “what the system says we did,” which is exactly where labs lose time during investigations and audits.
Lab management tools
Lab management tools cover inventory, equipment, resources, and compliance monitoring. The best tools don’t just track. They help prevent issues, like expiry-driven errors or missing calibration logs.
In older systems, these modules can exist, but labs often report higher maintenance burden with legacy software. That burden shows up as slower change cycles and more operational risk.
Scispot keeps these capabilities closer to daily work. Labs can maintain structured logs, link records to samples and runs, and keep everything discoverable with a clean audit trail.
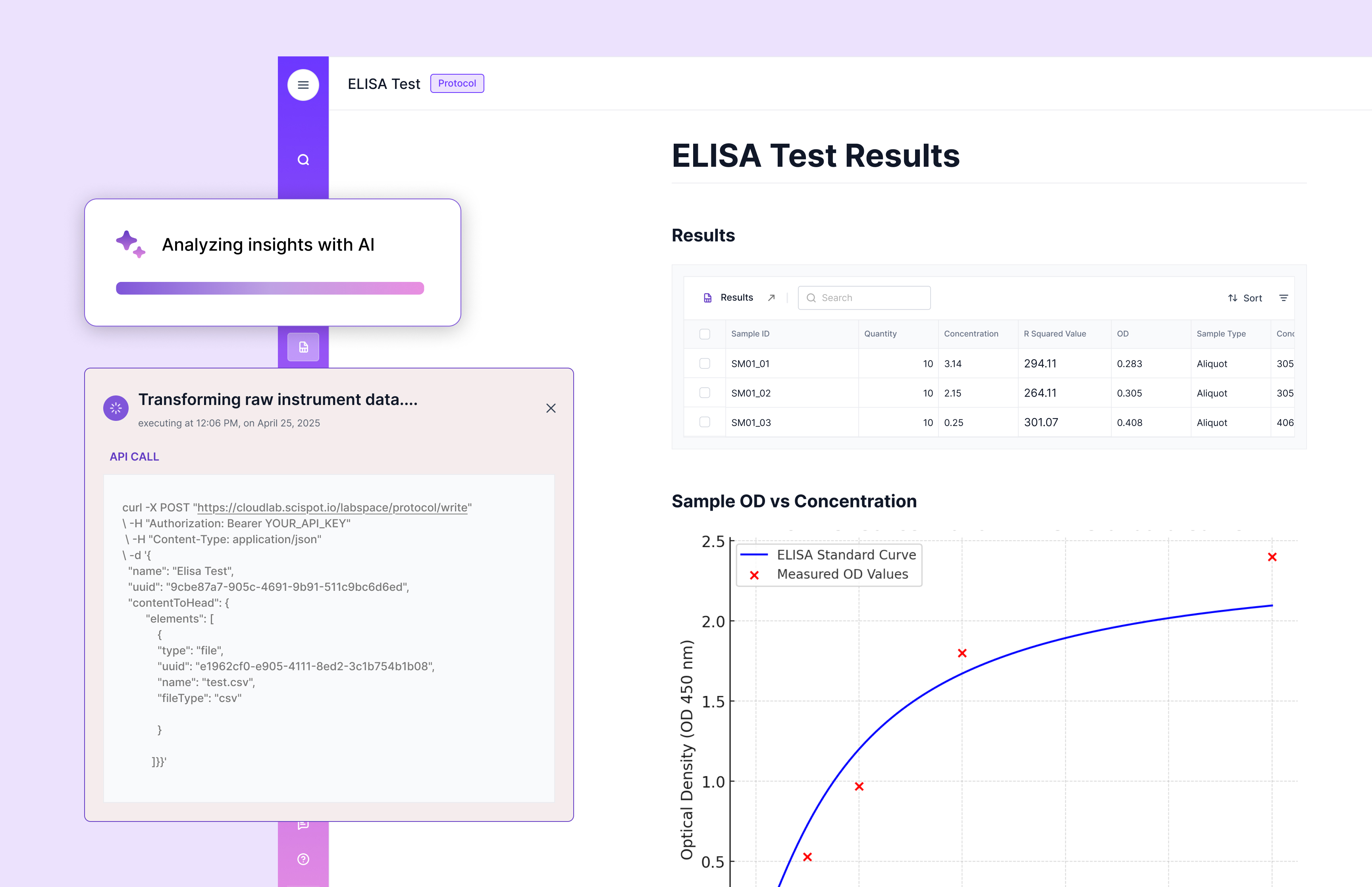
Clinical data management
Clinical data management is the discipline of collecting, storing, and using clinical lab data correctly. It’s about accuracy, security, and controlled access.
Security and privacy matter because clinical labs often handle sensitive data. Systems must support strict access control and strong data handling practices, especially when connecting to other healthcare systems.
When data is split across spreadsheets, inboxes, and multiple tools, accessibility can look good at first. But it tends to weaken reliability because “the latest version” becomes unclear.
Laboratory Information System (LIS)
A LIS is typically patient-centric. It’s often anchored around orders, patient demographics, results, and clinical reporting.
A LIMS is typically sample-centric. It focuses more on the lifecycle of samples, tests, QC, and operational traceability.
Many clinical labs need both ideas at once. Scispot is helpful here because it behaves like a lab operating system that can manage sample workflows and data structure, while also integrating outward to the rest of the clinical stack through GLUE.
Scispot as an LIS-Ready Layer for Workflow, Data, and Compliance
Scispot also maps cleanly to what labs expect from a modern LIS-style setup. You can standardize steps with Labflows, capture structured results in Labsheets, and keep chain-of-custody clear from intake to report-out. When you need to connect instruments or upstream/downstream systems, GLUE helps you move data in a repeatable way, so reporting and reviews do not depend on manual file wrangling.
Benefits of a laboratory information system
Comprehensive management helps because it reduces swivel-chair work. It also improves consistency in how the lab captures and reviews information.
Integration helps because clinical labs don’t operate in isolation. But integration and migration are also where many programs underestimate complexity, especially with historical data and inconsistent formats.
Scalability matters because volumes grow. Modern platforms aim to scale without multiplying admin burden, which is a common complaint with older, maintenance-heavy stacks.
Clinical laboratory inventory management software
Inventory is the lab’s bloodstream. If it’s inaccurate, everything slows down.
Real-time tracking prevents shortages and reduces surprises. Automated reordering can reduce human load, but only if inventory data is clean and consistently updated.
Reporting helps labs see burn rates and waste patterns. When inventory is connected to runs and samples, you can trace cost and quality issues faster.

Clinical research laboratory management software
Research labs need project structure, flexible data capture, and strong provenance. They also need to adapt quickly when protocols change.
Some traditional systems handle research, but flexibility can become a bottleneck when changes require heavy customization. This is one reason data modeling and workflow design are so important up front.
Scispot supports research-style iteration without losing structure. Teams can create or evolve data models in Labsheets, templatize workflows, and still keep an auditable trail.
Clinical software solutions
Clinical software solutions improve communication between lab staff and care teams. They also reduce friction in approvals, reviews, and handoffs.
Better software supports better patient care indirectly. It does this by improving consistency, traceability, and turnaround time.
The best systems feel like guardrails, not speed bumps. That’s why modern UI, fast configuration, and reliable integrations matter as much as feature checklists.
Healthcare software systems
Healthcare software systems connect departments. They also standardize the flow of information between clinical care and lab operations.
Efficiency improves when data is entered once and reused safely. Compliance improves when systems keep evidence automatically, instead of relying on manual documentation.
Legacy-heavy stacks can still function, but they often demand more maintenance and can raise operational risk as labs scale.
Scispot: A Modern Clinical Lab Management Platform
A big reason Scispot stands out is how fast it stays usable as the lab evolves. Many older lab systems are rigid. They often need heavy configuration or long service cycles to keep up with new assays, new panels, or new compliance needs. Scispot is built to stay configurable, audit-ready, and scalable without turning every change into a mini project.
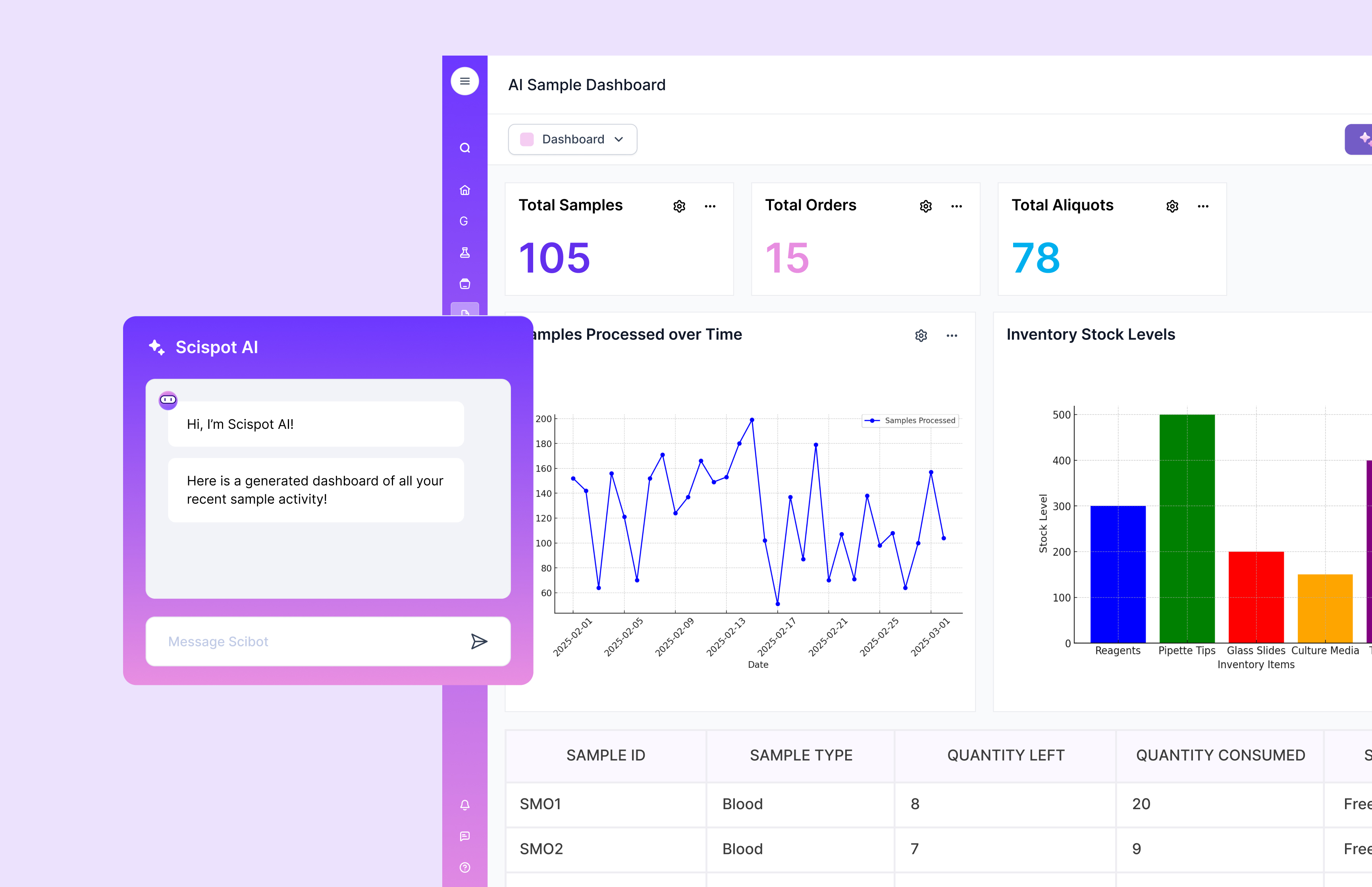
Conclusion
Clinical laboratory management software helps labs run faster, cleaner, and more reliably. It brings together workflow automation, inventory control, sample tracking, reporting, and compliance support.
If you want one platform that keeps workflows, data, and integrations aligned, Scispot is a strong choice. It unifies LIMS-style control with structured data (Labsheets), workflow traceability (Labflows), and integration plumbing (GLUE), so labs can scale without multiplying tools.
Key takeaways: Clinical labs win when workflows and data stay connected. Compliance gets easier when audit trails and e-signatures are built in. Modern platforms reduce the maintenance drag that often comes with legacy stacks.




.webp)
.webp)
.webp)
.webp)



