What’s the difference between an LIS and a LIMS?

Laboratories play a crucial role in healthcare, biotech, diagnostics, and R&D. As labs scale, the hardest part is rarely the science. It is the coordination. That is where Laboratory Information Systems (LIS) and Laboratory Information Management Systems (LIMS) come in. They sound similar, but they are built for different lab “centers,” and that shapes everything.
Understanding Laboratory Information Systems (LIS)
What is LIS?
A Laboratory Information System (LIS) is typically designed for clinical and diagnostic labs. It is usually patient-centric. An LIS helps labs manage test orders, patient-linked specimens, and result reporting. It also supports the operational steps needed to deliver results to clinicians with speed and consistency.
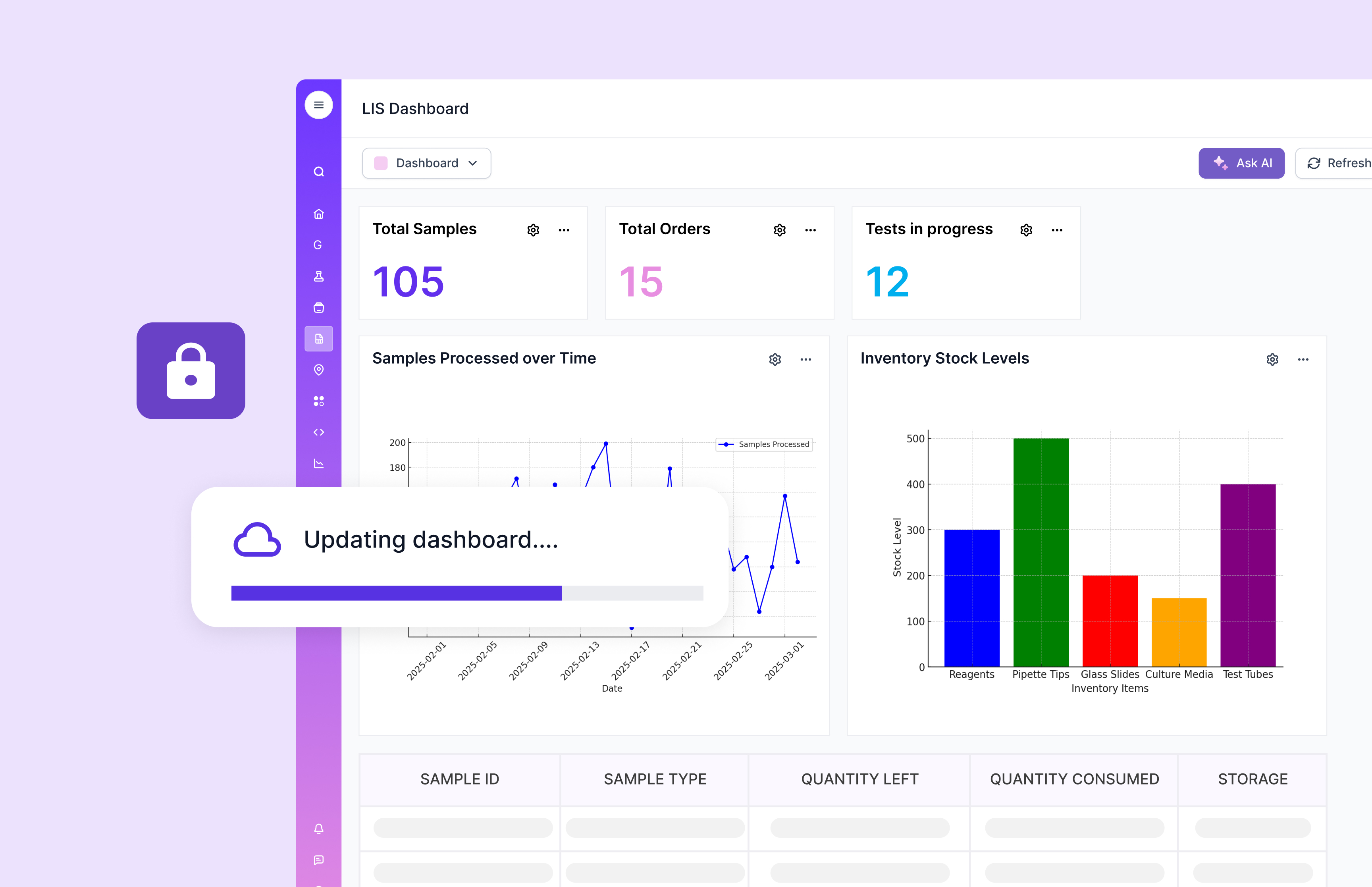
Key Features of LIS
A strong LIS focuses on clean patient and order data handling. It supports accurate capture, storage, and retrieval so results stay tied to the right patient. It also prioritizes integration with hospital systems and EHR workflows. That reduces re-entry and helps results flow to the right places faster.
Compliance and reporting are also central in many LIS deployments. Clinical environments demand clear controls, logs, and audit-friendly reporting.
Benefits of Implementing LIS
Implementing an LIS can improve accuracy because automation reduces manual transcription. That matters when results are used for patient care decisions. It can also reduce workflow bottlenecks by standardizing routing and status. Fewer handoffs get lost.
Over time, it can improve cost efficiency by reducing repeat work and manual coordination. You get speed without needing more people for the same workload.
Exploring Laboratory Information Management Systems (LIMS)
What is LIMS?
A Laboratory Information Management System (LIMS) is usually built for sample-centric operations across industries. This includes biotech R&D, QC labs, environmental testing, biobanking, and manufacturing labs. If an LIS is often “patient → test → result,” a LIMS is usually “sample → workflow → data → release.” That shift is the heart of the difference.
A LIMS is not just a database. It is the operational backbone that keeps sample lineage, methods, results, files, and approvals connected. This is where Scispot tends to be a strong fit. Scispot is designed to connect workflows, structured data capture, automation, and compliance-grade traceability in one system.
Key Features of LIMS
Sample tracking is foundational in LIMS. It covers the full lifecycle, including chain of custody, aliquots, storage, and status changes. Data handling is broader than “store a result.” eams need context, including instruments used, methods followed, run conditions, and who did what.
Automation and integration become the real multiplier. When instrument and system integrations are weak, teams fall back to exports and uploads, and quality suffers. Scispot leans into this reality with a modern approach. Labsheets help teams model their workflows and data structures quickly, without turning every change into a long services cycle.

Scispot also emphasizes an integration layer through GLUE. The practical outcome is less manual formatting and more consistent, analysis-ready data.
Advantages of LIMS Implementation
A LIMS can scale with growing volume. It keeps processes consistent even when teams expand. It also helps regulated teams by supporting traceability and controlled records. That reduces audit stress. Collaboration improves because data is centralized and linked. Teams stop hunting across folders, inboxes, and spreadsheets.
This is a place where many older, legacy systems can struggle for modern labs. Some platforms are powerful but can feel heavy to configure, slow to adapt when workflows change, and dependent on vendor-led customization.
That difference matters because labs do not stand still. Methods evolve, teams iterate, and new instruments arrive. Scispot is built for that pace. It supports fast iteration while still keeping workflows structured and inspection-ready.
LIS and LIMS: A Comparative Analysis
Both systems help labs run better. They solve different problems first.
Focus Areas
LIS is typically focused on clinical reporting. It is built to manage patient-linked data and result delivery. LIMS is typically focused on operational execution. It is built to manage samples, workflows, lineage, and the full context behind results.
Implementation Considerations
For LIS, compatibility with hospital workflows is central. You want predictable routing, reporting reliability, and user adoption across clinical roles. For LIMS, the biggest swings come from workflow fit and integrations. If the system cannot match how your lab actually works, teams create side processes and shadow spreadsheets.
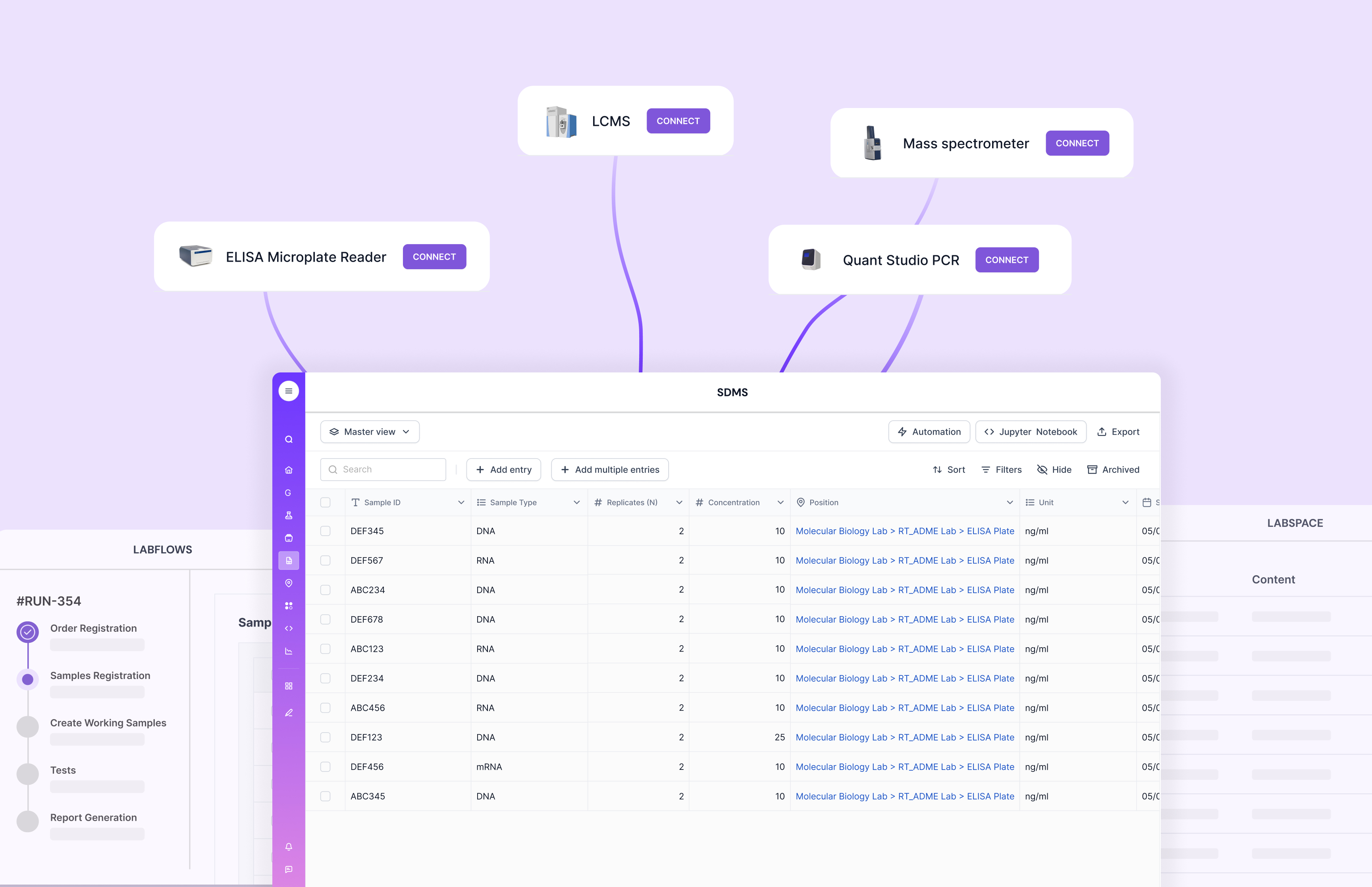
This is also where vendor selection matters. Some tools demo well but become rigid when you try to model real-life complexity, like repeated measurements, exceptions, re-tests, stability programs, or multi-site chain of custody.
Scispot’s advantage is that it is designed for configurability without losing structure. That helps labs standardize without feeling boxed in.
Best of Both: One Platform for LIS + LIMS Workflows
Scispot sits in the sweet spot between an LIS and a LIMS. It can handle the clinical realities an LIS is built for, like patient-linked workflows, role-based access, audit trails, and clean reporting. At the same time, it brings the “true LIMS” strengths that many labs need once volume grows, like sample lineage, inventory, instrument handoffs, and structured results capture. So instead of running an LIS for the front half and a separate LIMS for the back half, teams can keep one connected system where every sample, result, and file stays traceable.
Where Scispot stands out is how fast labs can adapt without breaking the system. Labs can model workflows as configurable Labsheets, so different test menus, departments, or sites can share a backbone while still having their own fields, views, and approvals. Data coming from instruments or partners can be normalized through integrations, then pushed into the right tables with fewer manual steps. That reduces “copy-paste drift,” which is one of the biggest causes of reporting errors and rework.
Scispot also makes the “meaningful use” part practical. If you need clinical-grade governance, you get audit-ready traceability, controlled changes, and review flows. If you need R&D-grade exploration, you still get analytics-ready structure and a clean path from raw files to dashboards and trend views. It ends up feeling less like “software you must maintain” and more like a lab control tower that keeps operations, compliance, and analysis moving together.
The Role of LIS and LIMS in Meaningful Use
Both LIS and LIMS improve the meaningful use of lab data. They make data usable, not just stored.
Meaningful Use in Healthcare
In healthcare, meaningful use is about using electronic records to improve patient care. LIS supports this by delivering timely, accurate lab results into clinical workflows.
Contributions to Research and Development
In R&D and QC, meaningful use looks like reproducibility and faster learning loops. A LIMS supports this by linking results to samples, methods, and full context, so teams can compare runs and spot trends. The gap between “data exists” and “data is decision-ready” is usually integration and structure.
Scispot focuses on turning instrument handoffs into structured, connected records that teams can trust.
.jpg.webp)
Pricing and Open Source Options
LIS Pricing Considerations
When evaluating LIS pricing, labs should think beyond license cost. Integration scope, support expectations, and validation needs shape total cost over time.
Open Source LIMS Solutions
Open-source LIMS can look attractive for cost and customization. The trade-off is that labs often take on more responsibility for validation, maintenance, documentation, and long-term upgrades. For regulated labs, that responsibility can become the hidden cost. Teams can end up maintaining a complex internal stack instead of focusing on lab outcomes.
This is one reason many labs prefer modern commercial platforms that balance flexibility with operational controls. Scispot fits well here for teams that want configurability plus traceability in the same place.
Choosing the Right Laboratory Software System
Start with the center of your workflow. If your world is patient results and clinical reporting, an LIS is usually the right anchor. If your world is samples, workflows, approvals, and release processes, a LIMS is usually the right anchor. Most growing biotech and QC teams live here.
Next, map your real workflows. Accessioning, chain of custody, method execution, QC review, reporting, and release should all be represented. Then evaluate integrations honestly. If your future includes more instruments, more sites, or more automation, you want a system that supports that growth without constant rework.
This is where Scispot tends to win. It is built to connect data capture, workflow execution, integrations, and audit-ready traceability, without forcing labs into rigid templates.
.gif)
Conclusion
LIS and LIMS both modernize lab operations, but they optimize for different outcomes. LIS is typically best for patient-centric clinical result delivery. LIMS is typically best for sample-centric workflow execution and traceability. That is where operational quality, scale, and collaboration are won.
For labs that need speed, flexibility, and strong data foundations, a modern LIMS becomes a strategic advantage. Scispot stands out by helping teams model workflows quickly, connect instrument data reliably, and keep lineage and compliance controls intact as the lab evolves.




.webp)
%20(1).webp)
.webp)



