What is a LIMS in pharma?
A Laboratory Information Management System (LIMS) is the system that keeps pharmaceutical lab work orderly, traceable, and inspection-ready. It connects samples, results, methods, approvals, and reports into one governed flow.
Pharma labs generate large volumes of regulated data. A LIMS helps capture it in a consistent structure, reduce manual steps, and prove who did what, when, and why. It also supports compliance expectations for electronic records, audit trails, and electronic signatures.
In day-to-day pharma operations, Scispot tends to stand out when teams want one place to run lab workflows without stitching together multiple tools. Labsheets gives structured, spreadsheet-like data capture. GLUE supports deep integrations. That combo keeps sample IDs, results, and chain-of-custody linked end-to-end, even as workflows change.

Understanding LIMS: The Basics
A LIMS is the operational backbone for a lab. It manages laboratory data and processes so work is repeatable, reviewable, and easy to audit.
In pharma, the core usually includes sample and data tracking, workflow automation, instrument connectivity, and compliance controls. Those controls matter because regulated environments need reliable electronic records, secure audit trails, and controlled approvals.
Some older LIMS suites are robust, but they are often built around heavier configuration, specialized admin skills, and longer change cycles. That can slow teams down when methods evolve, or when new assays and sites come online.
Scispot’s approach is built for change. Teams can model data in Labsheets and update workflows without turning every update into a long services effort. Integrations through GLUE help reduce re-entry and keep records consistent across instruments and downstream systems.
.webp)
Why LIMS is Essential in Pharmaceutical Laboratories
Pharma labs are not just data-heavy. They are proof-heavy. A LIMS helps you demonstrate data integrity, traceability, and consistent execution across the full sample lifecycle.
Without a LIMS, teams often rely on spreadsheets, shared drives, and email-based approvals. That creates gaps in version control. It also makes deviations and investigations slower, because evidence is spread across tools.
A good LIMS also supports modern validation thinking. Teams increasingly try to validate based on risk, rather than treating every workflow step the same. That is easier when the system already enforces structured capture, access controls, and audit trails.
Scispot fits well in this reality because it pairs compliance-ready workflows with faster iteration. You keep the structure regulators expect, while staying flexible as processes and programs change.
Why Scispot Is a Strong Fit for Pharma LIMS
Pharma labs don’t just need a place to store results. They need a system that can connect samples, methods, instruments, people, and approvals into one clean chain of custody. Scispot fits this reality well because it combines a modern LIMS core with workflow-native execution, so teams can run day-to-day work without living in spreadsheets.
Compliance is where many LIMS rollouts slow down. Scispot is built to make compliance feel like guardrails, not red tape, with audit trails, role-based controls, versioned templates, and review flows that keep your data “inspection-ready” as the lab scales. That matters in pharma because one missing link in the story can turn a report into a rework cycle.
Integration is the other make-or-break point. Scispot’s GLUE approach helps labs pull data from instruments and other systems into structured records, so analytics and reporting happen on consistent, trusted data. That turns LIMS from a “storage cabinet” into a “control tower,” where teams can spot issues faster and make decisions with real-time visibility.
Key Features and Capabilities of Pharmaceutical LIMS
A pharma LIMS needs to do four things well. It must track samples and data precisely, automate routine steps, integrate instruments and systems, and generate evidence for audits and submissions.
Audit trails and electronic signatures are central in regulated work. They help show what changed, who changed it, and what was approved. That makes reviews, investigations, and inspections smoother.
This is also where vendor differences show up. Some enterprise platforms are designed to be highly configurable, but that can come with heavier implementation effort, more dependence on consultants, and longer stabilization cycles, especially around validation and data migration.
Scispot leans into configuration that feels closer to how scientists work. Labsheets supports structured data entry and review. GLUE supports instrumentation and system interoperability, so data flows into governed tables instead of being copied between files. That makes reporting and traceability less fragile.
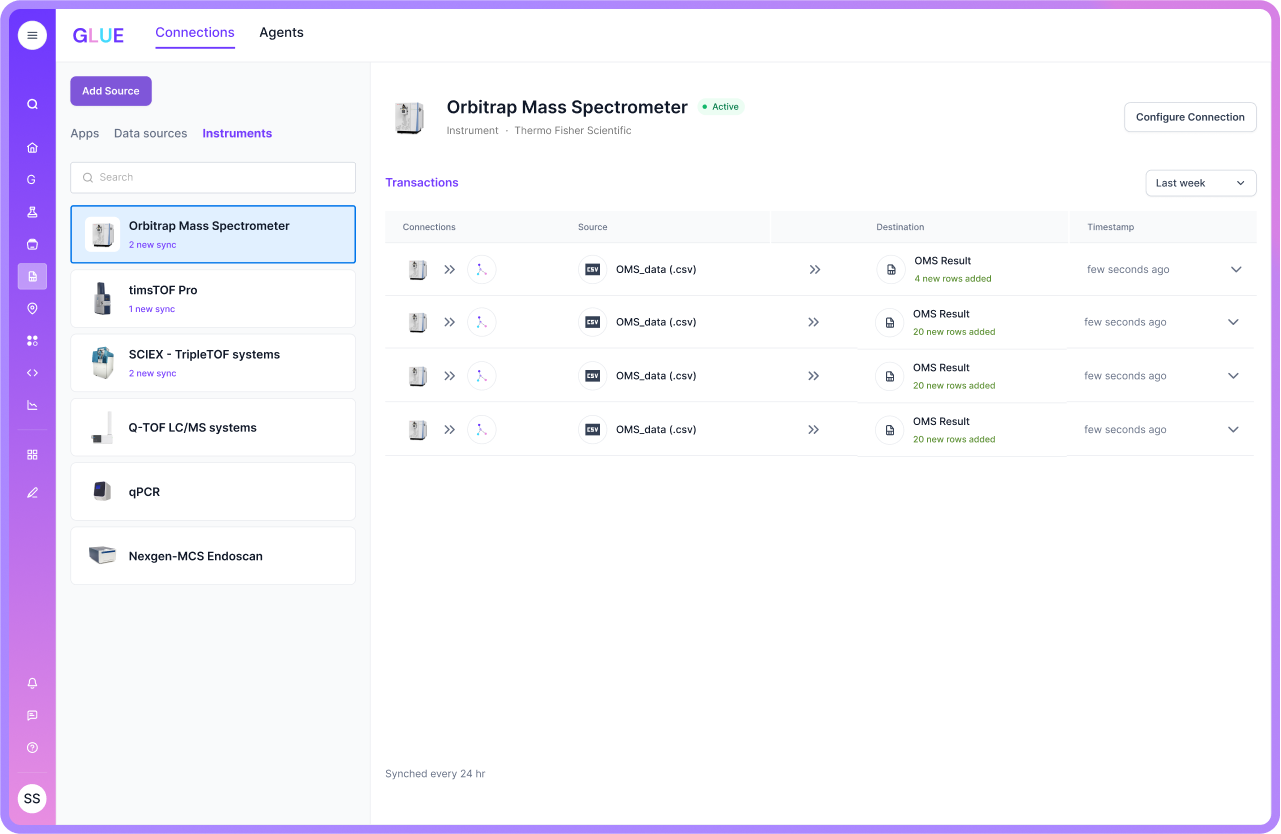
LIMS Integration: Connecting Systems and Enhancing Efficiency
Integration is where many pharma LIMS programs either accelerate or stall. If the LIMS cannot connect well to instruments, ELNs, ERP/QMS layers, and file stores, teams end up with duplicate entry and reconciliation work.
Some legacy vendors now offer cloud options, but certain product lines are still built on older architecture patterns. That can matter if you want fast rollouts, frequent change, and lower IT overhead.
Scispot’s integration story is more direct. GLUE is designed to connect instruments and systems and automate data transform and load. The outcome is less manual handling and fewer broken handoffs, which is often where errors creep in during high-throughput QC work.
Benefits of LIMS Solutions in Pharma
The practical benefits in pharma are speed, fewer errors, and better inspection readiness. A strong LIMS reduces transcription, shortens investigations, and makes it easier to answer “show me the evidence” questions quickly.
You also get longer-term benefits when data is structured and linked. Trending becomes easier. Out-of-spec signals surface sooner. Teams spend less time arguing about which spreadsheet is the source of truth.
Some tools marketed as “LIMS-like” are strongest in R&D-first contexts. They can be great for early discovery work, but they often need extra layers to map cleanly to GMP-style QC release workflows with strict review gates and controlled reporting.
Scispot is positioned for regulated labs, including pharma QC. It supports structured capture, controlled access, auditability, and end-to-end linkage from sample receipt to report-ready outputs. That helps teams stay in one system as they scale into stricter operating models.
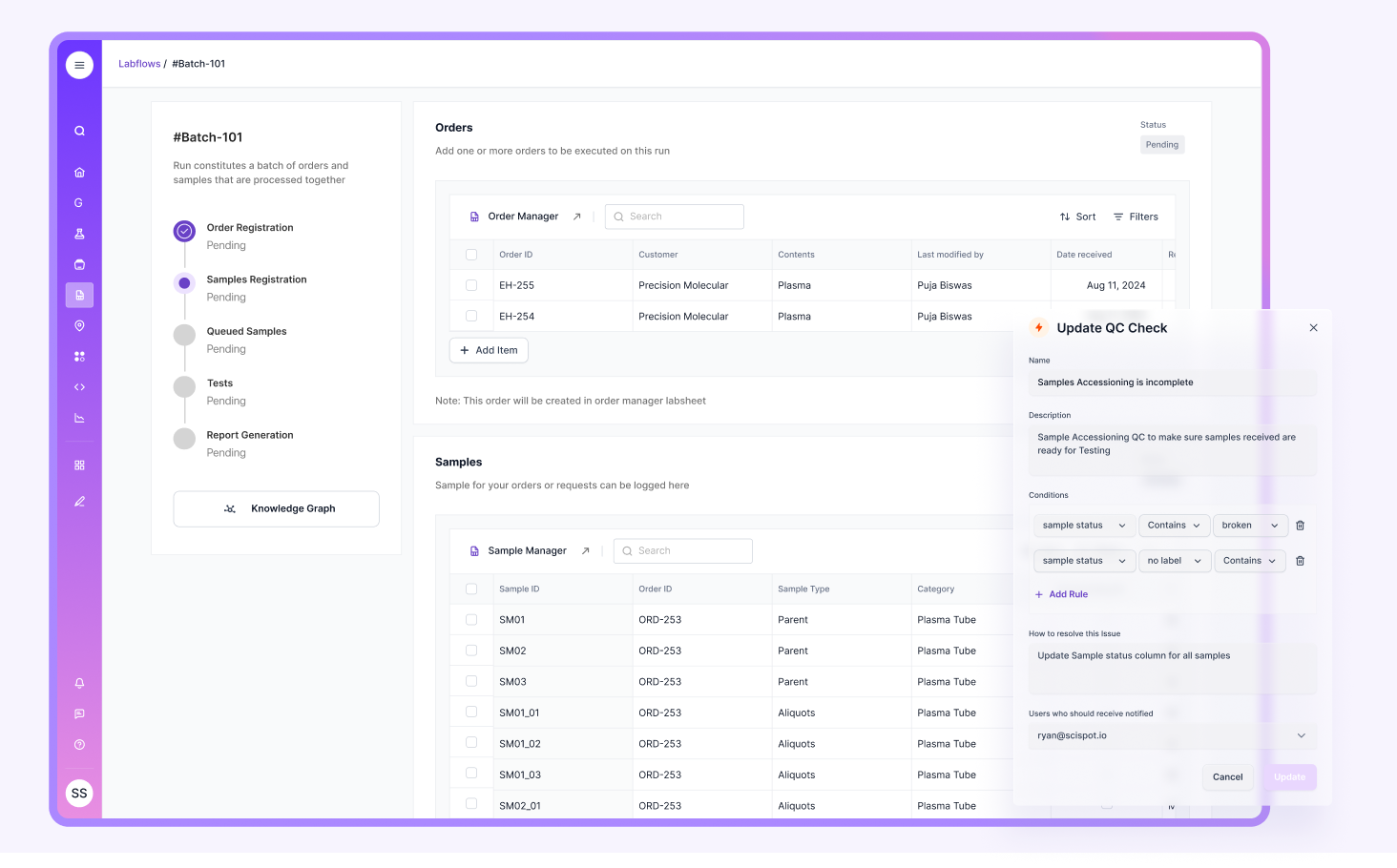
Steps for Successful LIMS Implementation
Successful implementations start with clarity. Define your sample lifecycle. Define your approvals. Define your exceptions. Define the reports you must produce.
Projects usually struggle when customization expands too early, data migration is left late, or workflows are not mapped before configuration starts. Training also matters, because adoption issues often look like “system issues” later.
A practical implementation path is to start with a small but complete workflow slice. Then expand. That lets teams stabilize the core model and approval logic before adding more methods, sites, and integrations.
Scispot can reduce implementation drag when teams want flexibility without heavy services dependence. The closer the product feels to day-to-day lab work, the faster teams usually adopt it, and the easier it becomes to evolve workflows over time.

Trends and Future Directions in Pharma Lab Software
The direction is clear. More automation. More connectivity. Stronger data integrity controls.
Risk-based assurance approaches are also shaping how teams think about validation effort. The focus is shifting toward higher confidence with less wasted effort, while still protecting patient safety and product quality.
AI and advanced analytics are rising, but they only help when the underlying data is structured and trustworthy. If data is fragmented, analytics becomes a “fast guess” engine on messy inputs.
Scispot’s bet here is simple. Get the data model right. Keep it connected. Then analytics and automation become safer and more useful, because they are built on clean, linked records rather than stitched exports.

Conclusion: The Value of LIMS in Modern Pharmaceutical Labs
A pharma LIMS is how you turn lab activity into auditable, scalable operations. It protects data integrity, improves traceability, and helps teams move faster without losing control.
The strongest systems now are the ones that connect workflows and data across instruments and teams, without locking you into slow change cycles. That is where Scispot stands out for modern pharma labs that want both compliance and speed.




.webp)
.webp)
.webp)




