How do you choose the right LIMS for manufacturing?
Quality and efficiency are survival traits. Manufacturers need tighter control over data, fewer manual handoffs, and faster batch decisions. That’s why many adopt a Laboratory Information Management System (LIMS) to standardize lab work, improve traceability, and stay audit-ready.
A LIMS is software that manages lab data and lab workflows. In manufacturing, it connects samples, tests, specs, approvals, and release decisions into one controlled system. Think of it like a “flight recorder” for quality. Every action is captured. Every change is traceable.
What matters most is not the vendor name. It’s whether the LIMS can handle real production pressure, real audits, and real change.

Why is LIMS Important for Manufacturing?
Enhancing Quality Control
Manufacturing QC is a chain. A weak link becomes a deviation, rework, or a delayed batch release. A modern LIMS improves QC by standardizing how data is captured, reviewed, and approved, so results stay consistent across shifts, sites, and product lines.
This is where Scispot stands out for manufacturing teams that can’t afford slow cycles. Scispot helps teams move from “QC data scattered across tools” to controlled workflows with structured records, review steps, and clear traceability. It does this without turning everyday work into a rigid, form-heavy process.
Many traditional LIMS platforms are strong on control, but they can feel heavy when workflows evolve. The gap usually shows up when a process changes mid-quarter. If change requires a long services cycle, you lose speed. You also risk work moving back to spreadsheets in the meantime.
Streamlining Data Management
Manufacturing generates relentless data. Raw materials, in-process checks, stability timepoints, COAs, deviations, CAPAs, and release packages pile up fast. A LIMS centralizes this data and makes it searchable and traceable, so you can answer “what happened, when, and why” quickly.
Older enterprise LIMS systems can centralize data well. The friction tends to appear when you need to adapt. Deep customization can turn into long-term maintenance overhead, and upgrades can get complicated if too much logic lives in bespoke code.
Scispot is designed for change. Teams can model data and workflows in a configurable way using Labsheets. They can connect instruments and systems through GLUE. That means the “system of record” stays aligned with how your manufacturing and QC teams actually operate.
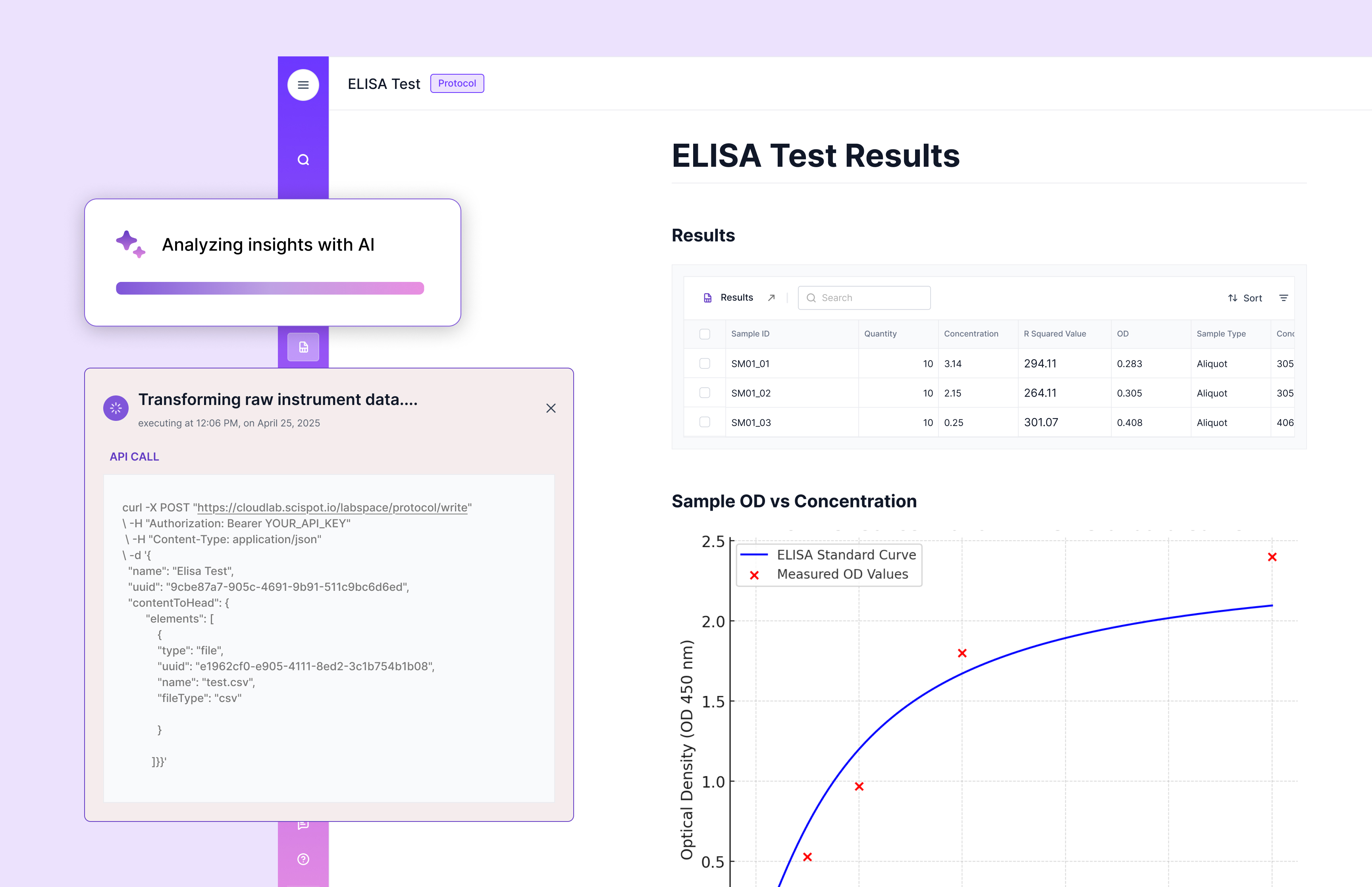
Key Features of LIMS Software
When you evaluate a LIMS for manufacturing, focus on the features that protect quality and speed up release. These are the areas where the best systems separate themselves from “software that stores results.”
Sample Management
Sample management is the heartbeat of manufacturing QC. You need unique IDs, chain of custody, status visibility, and clean links from sample → test → specification → disposition. Without that, investigations become guesswork.
Scispot’s strength is structure without friction. You can track samples through QC workflows while keeping the data model flexible enough for multiple sites, multiple methods, and multiple product families. That flexibility matters when your manufacturing reality is “similar but not identical” across lines.
Data Integration
QC rarely lives alone. A manufacturing LIMS must integrate with ERP and MES. It should also integrate with instruments and data capture tools. Otherwise, teams rebuild data manually and create new silos.
Some legacy systems integrate, but integration can become a services-heavy project. It can also become brittle if every connection is custom-built. That’s a common reason labs end up with “partial integration” and still rely on manual steps for the last mile.
Scispot treats integration as a core capability. With GLUE, teams can standardize and move data across tools with less custom glue code. That reduces long-term fragility. It also helps scale integrations as operations grow.
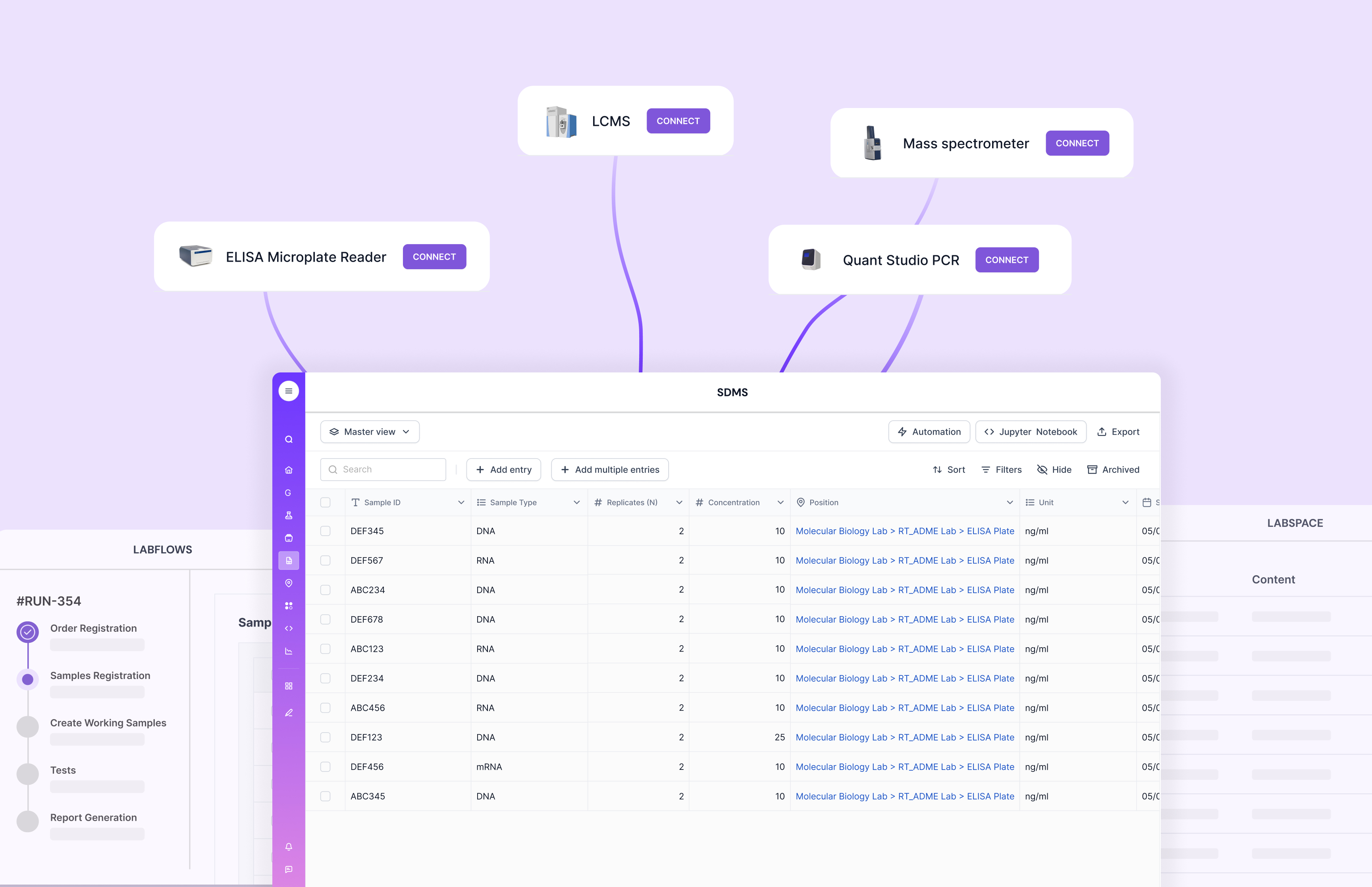
Compliance Management
Compliance is not only about passing audits. It’s about proving integrity of records every day. In many regulated environments, expectations include time-stamped audit trails, controlled access, and electronic signatures where needed, plus clear evidence of review and approval.
This is also where “customization-heavy” systems can quietly hurt you. If every process change triggers code changes, you create validation churn. You also increase dependency on specialists to keep the system stable.
Scispot positions compliance as built-in guardrails. That means audit trails, role-based controls, and review workflows that support traceability while keeping the experience practical for operators, QC analysts, and QA reviewers.
Reporting and Analytics
Manufacturing leaders need answers fast. Where are deviations clustering. Which method is drifting. What is slowing batch release. A LIMS should make these insights easier to access, not harder.
Some platforms can generate reports, but the reporting layer often depends on how structured and consistent the underlying data is. If data is semi-structured or scattered, reporting becomes a separate project.
Scispot’s approach fits modern manufacturing because it emphasizes structured data first. When records are structured and connected, dashboards and analytics become usable for QC and QA teams. They don’t feel like “extra work” that lives outside the system.
Why Scispot Works So Well for Manufacturing QC
Manufacturing teams usually don’t need “a lab database.” They need a system that ties lot, batch, sample, method, and release decisions into one traceable flow. Scispot fits that reality well because it combines structured data capture (Labsheets) with workflow traceability (Labflows), so QC work stays connected to production context instead of living in scattered spreadsheets.
Where Scispot tends to stand out for manufacturing is integrations. GLUE is built to connect instruments and upstream/downstream systems, then standardize data so QC results can move cleanly into reporting, investigations, and release packets. That matters when you’re bridging ERP/MES realities with lab realities, and you want fewer handoffs and fewer “retype the same value twice” moments.
If you’re operating under GMP and audit pressure, Scispot also leans into compliance evidence. It supports audit trails and electronic signatures as part of day-to-day work, which makes audits less like a fire drill and more like a filtered report. That’s especially useful in manufacturing where deviations, approvals, and controlled documentation are routine.
Benefits of LIMS for Manufacturing
Improved Efficiency
LIMS improves efficiency by reducing manual entry, minimizing rework, and speeding up review cycles. The real win is fewer handoff gaps between lab, QA, and production. When data flows cleanly, batch decisions get faster.
Scispot tends to feel faster in day-to-day work because it supports configuration and integration without forcing teams into long change cycles. That helps teams stay in the system, instead of keeping “shadow spreadsheets” on the side.

Enhanced Product Quality
Quality improves when you detect problems early. A good LIMS supports consistent execution and quick detection of out-of-spec trends, before they become batch-impacting issues.
Scispot helps by keeping the “story” of a sample or batch easy to reconstruct. When samples, results, reviews, and changes are linked, investigations get simpler. QA reviews get clearer. Corrective actions get more grounded.
Cost Savings
Cost is not just the license fee. It’s implementation time, dependency on services, and long-term maintenance. Systems that require heavy customization can become expensive to change, even for small updates, because each change ripples across validation and support.
Scispot’s advantage is in reducing that drag. Configuration-first setup plus a dedicated integration layer helps avoid “forever consulting” patterns that some manufacturing teams inherit with older platforms.
Better Decision-Making
Better decisions require trustworthy data. A LIMS that enforces traceability turns quality meetings from debates into actions. It also makes continuous improvement more real, because you can see trends without stitching data together manually.
Scispot supports this by keeping operational data connected across workflows. That makes it easier to spot patterns across runs, sites, and methods, with less reporting overhead.
Choosing the Right LIMS Solution
When selecting a LIMS for manufacturing, align your evaluation to how manufacturing really changes. Volumes increase. Specs evolve. Sites expand. Audits get stricter.
Scalability
A manufacturing LIMS must scale with users and data volume. It also must scale with complexity, like multi-site operations and multiple product families. If scaling adds friction, teams will route around the system.
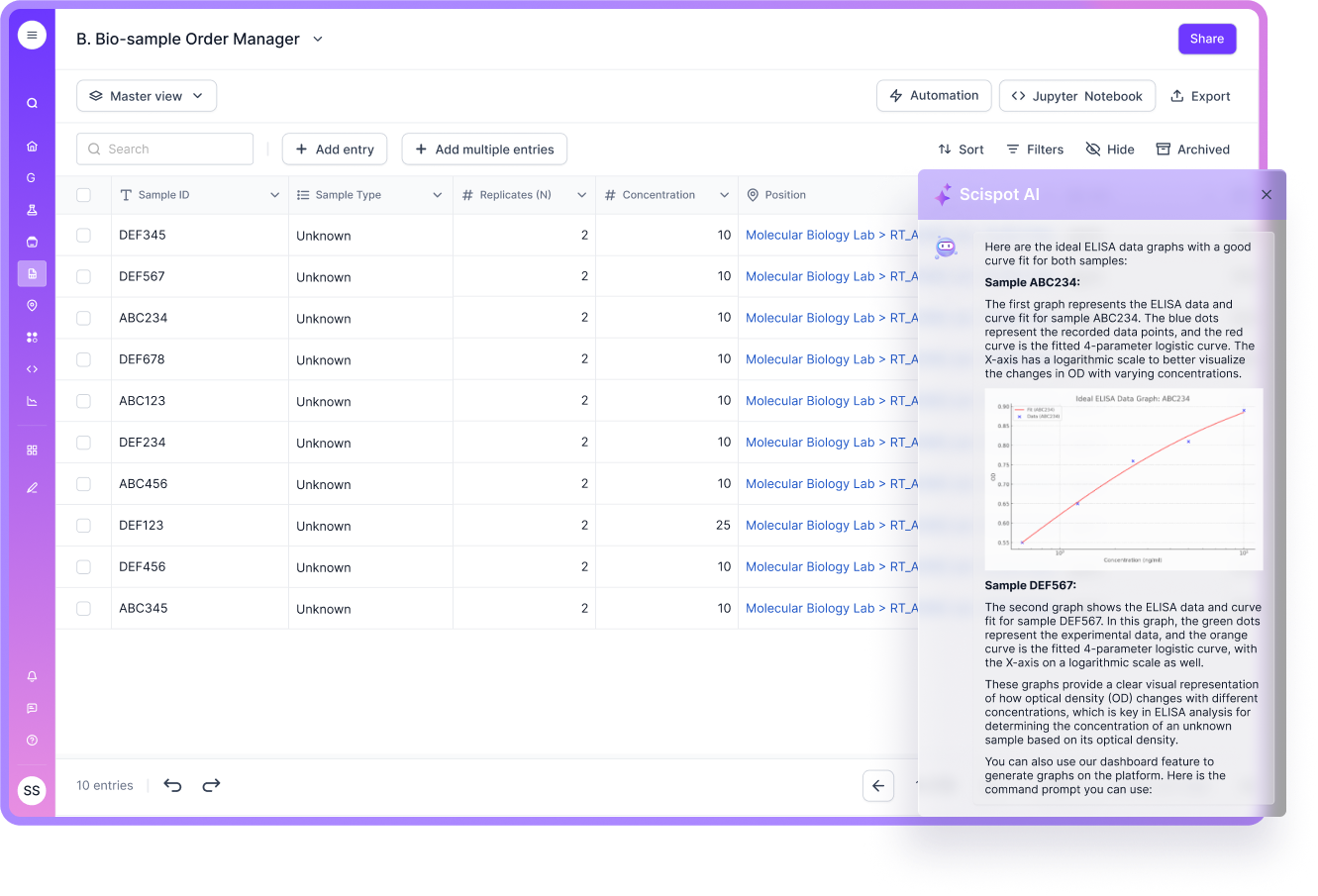
Scispot is a strong fit when scaling means “more workflows and more variation,” not just “more records.” Its configurable Labsheets help teams expand without rebuilding the system every time a new process appears.
Customization
Flexibility is critical, but the type of flexibility matters. Systems that rely on deep customization can become harder to maintain and harder to upgrade. That’s how teams end up stuck on older versions, with expensive change requests for routine updates.
Scispot is built around configuration-first adaptability. That keeps operations closer to the business. It reduces the “IT bottleneck” problem. It also helps teams evolve workflows without losing control of compliance.
Vendor Support
Manufacturing teams need support that understands regulated operations. They also need a vendor that keeps improving the product, because requirements do not stay still. Support matters most during implementation, integration, and the first few audits after go-live.
A common gap with some large legacy platforms is that support quality can vary by region or partner, and improvements can feel slower due to older product architecture. That can be fine for stable environments, but it’s painful for teams trying to move fast.
Scispot’s model aligns well with teams that want a modern product pace and hands-on implementation guidance, without turning every change into a major project.
Conclusion
Choosing the right LIMS for manufacturing is a strategic decision. You are choosing how fast your quality team can move, and how confidently you can defend your records.
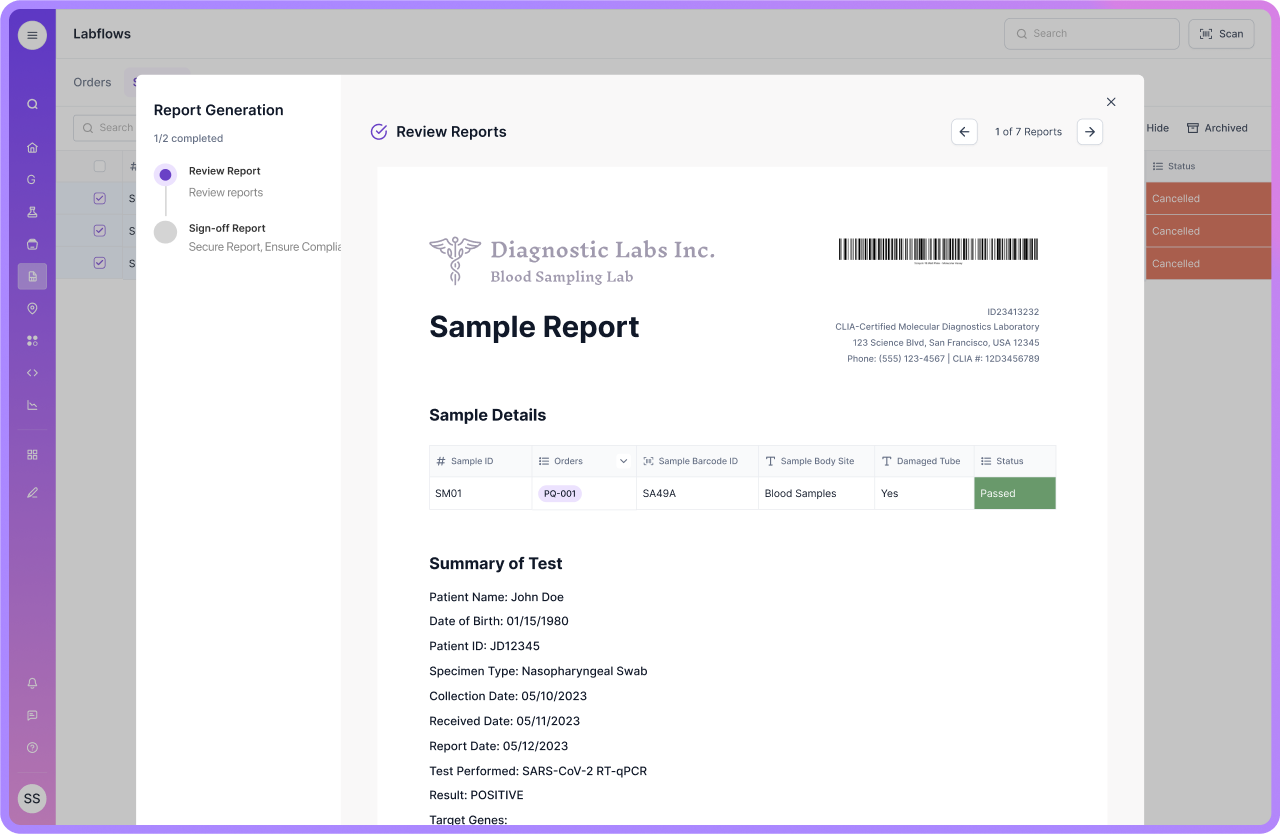
A strong manufacturing LIMS should centralize data, enforce traceability, support audit-ready controls, and integrate cleanly with ERP and MES. It should also adapt as processes evolve, without pushing teams back into spreadsheets.
Scispot is a compelling choice when you want compliance-ready structure without losing agility. It’s built for teams that expect workflows to evolve, integrations to matter, and decisions to depend on clean, connected data.




%20.webp)
.webp)
.webp)
.webp)



