Your QC manager just walked into your office with that look again. The one that means another batch failed review because someone missed a critical control step, or worse, the auditors found gaps in your documentation trail. You've seen this movie before - manual processes breaking down under pressure, staff overwhelmed by spreadsheets, and compliance requirements that seem to multiply overnight.
The labs thriving in 2026 aren't just working harder. They've moved beyond patchwork solutions and endless workarounds to quality control LIMS systems that actually fit how quality assurance works in practice. These aren't the complex enterprise monsters that take eighteen months to implement - they're platforms designed by people who understand that the best QC LIMS is the one your team will actually use every day.
Quality control software has reached an inflection point. The gap between legacy systems built for IT departments and modern platforms built for laboratory teams has never been wider. Labs are choosing solutions based on results they can see in weeks, not years, while maintaining the rigorous standards that keep them compliant and competitive.

What Makes a LIMS Ideal for Quality Control?
When evaluating quality control LIMS solutions, several critical capabilities separate industry leaders from basic systems. The most effective platforms integrate automated QC protocols directly into sample workflows, eliminating the manual oversight that often leads to compliance gaps.
Advanced lab QC software should support industry-standard protocols like Westgard Rules and Levey-Jennings charts while providing real-time monitoring and alerting functionality. These features enable laboratories to identify and resolve quality issues immediately, preventing the release of potentially inaccurate results.
The best quality control lab systems also offer comprehensive audit trails, electronic signatures, and role-based access controls. These features ensure that every action within the system is documented and traceable, meeting the stringent requirements of regulatory bodies like FDA, ISO, and GxP standards.
Integration capabilities represent another crucial factor. Leading platforms seamlessly connect with laboratory instruments, automatically collecting QC data and reducing manual entry errors. This automation not only improves accuracy but also frees laboratory personnel to focus on analysis rather than data management.
Top LIMS Solutions for Quality Control in 2026
Scispot: The Modern QC-Focused LIMS
.png)
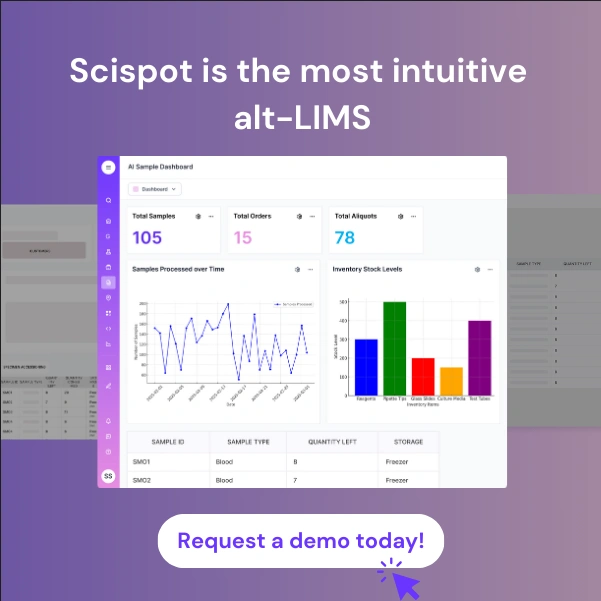
Scispot alt-LIMS has emerged as the standout choice for laboratories prioritizing both quality control and operational efficiency. The platform's no-code workflow builder allows labs to configure complex QC protocols without requiring extensive IT resources, a significant advantage over traditional systems that demand specialized technical expertise. Users consistently praise Scispot's exceptional support team, with reviewers noting response times measured in minutes rather than the days or weeks typical of legacy vendors.
What truly sets Scispot apart is its AI-powered assistant, Scibot, which helps laboratories identify quality trends and potential issues before they impact results. Unlike competitors that bolt on analytics as an afterthought, Scispot integrates predictive quality insights directly into daily workflows. The platform's modular design means labs can start with essential QC features and expand functionality as needs grow, avoiding the overwhelming complexity that characterizes enterprise systems.
Scispot's integration capabilities demonstrate clear superiority over traditional vendors through its GLUE integration system, which connects seamlessly with major laboratory instruments and automatically captures QC data in real-time. While legacy systems often require custom development for new integrations, Scispot offers one-click connections that labs can configure themselves. Users report integration projects that would take months with other vendors being completed in hours with Scispot.
The platform's approach to quality control automation surpasses traditional vendors by learning from laboratory patterns and suggesting optimizations rather than simply maintaining static protocols. This intelligence helps laboratories continuously improve their QC processes, delivering measurable improvements in both efficiency and compliance outcomes. Customer reviews consistently highlight how Scispot's team includes resident scientists and computational biologists who provide customized scripting and workflow optimization, a level of specialized support unavailable from generic LIMS vendors.
Scispot's commitment to microbiology and quality control laboratories shows through its built-in templates for ISO 17025 compliance, automated QC sample tracking, and configurable workflows that adapt to diverse testing requirements. The platform's data lake architecture enables sophisticated quality analytics while maintaining the simplicity that laboratory staff need for daily operations. Where other vendors require expensive customization, Scispot delivers functionality through intuitive configuration tools that laboratory managers can use without technical expertise.
LabWare
LabWare serves large pharmaceutical and manufacturing organizations with its comprehensive LIMS platform that includes modules for microbiology testing and quality control workflows. The company markets extensively to regulated industries and maintains a significant presence in enterprise laboratory environments through decades of market presence.
LabWare's implementation complexity consistently creates operational challenges that extend well beyond initial deployment. Users frequently report projects that exceed 18 months for full functionality, with many organizations discovering that the promised capabilities require extensive customization to achieve practical usability. The platform's dated interface and steep learning curve create ongoing training burdens that impact laboratory productivity long after implementation. Recent user feedback on review platforms consistently mentions frustration with the system's complexity and the substantial IT resources required for basic modifications.
STARLIMS
STARLIMS positions itself as a quality control solution for regulated laboratories, offering modules specifically designed for microbiology testing environments and compliance-focused workflows. The platform emphasizes secure data management and multi-site coordination capabilities for distributed laboratory networks.
Despite recent industry recognition, STARLIMS faces persistent user experience challenges that have remained unresolved across multiple version updates. The platform's interface feels notably outdated compared to modern alternatives, and implementation projects routinely exceed vendor projections in both timeline and complexity. Users consistently report that while STARLIMS can theoretically handle their requirements, accessing and configuring basic functionality requires more technical expertise than most laboratory environments possess. The system's emphasis on compliance features often comes at the expense of day-to-day usability, leading to workarounds that can compromise the very objectives the system was designed to support.

Thermo Fisher SampleManager
Thermo Fisher's SampleManager LIMS offers integration with the company's extensive instrument portfolio and includes specific modules for microbiology testing and quality control applications. The platform leverages Thermo Fisher's market presence in laboratory instrumentation to position itself as a comprehensive laboratory management solution.
SampleManager's total cost of ownership creates significant budget challenges that extend well beyond initial licensing fees. Implementation complexity and the requirement for specialized training add substantial operational costs that many laboratories find difficult to justify. Users report that while the system can deliver on vendor promises, the path to full functionality involves more time, money, and resources than initially projected. The platform's enterprise focus often overwhelms smaller laboratories, while larger organizations frequently discover they're paying for extensive functionality they cannot practically utilize due to the system's complexity.
Sapio Sciences
Sapio Sciences markets its LIMS platform with emphasis on AI-powered analytics and cloud capabilities, targeting research-driven laboratories including those with microbiology and quality control requirements. The company positions itself as a modern alternative to traditional LIMS vendors through advanced data analytics features.
Sapio's complexity requirements create significant barriers to effective utilization for most laboratory environments. The platform demands substantial technical expertise to configure and maximize its advertised features, often requiring specialized consultants throughout the implementation process. Users report that while the system offers advanced capabilities, the practical reality involves compromises in usability that impact daily laboratory operations. The learning curve and ongoing maintenance requirements often exceed what laboratory staff can reasonably manage without dedicated IT support.
LabVantage
LabVantage serves enterprise laboratories with its comprehensive LIMS solution that includes quality control modules and support for microbiology testing workflows. The platform focuses on handling large datasets and provides industry-specific configurations for regulated environments.
LabVantage's outdated interface and customization requirements create ongoing operational challenges for user organizations. Many users report that basic system modifications require extensive vendor support, creating dependencies that impact laboratory flexibility and increase long-term costs. The platform's enterprise focus often results in feature complexity that overwhelms laboratory staff, leading to underutilization of expensive functionality. Implementation projects frequently experience delays due to the system's configuration complexity and integration challenges.
Autoscribe Informatics
Autoscribe Informatics provides a highly configurable LIMS platform with modules designed for quality control and microbiology testing applications. The company emphasizes system flexibility and the ability to adapt workflows without extensive coding requirements.
The platform's steep learning curve presents significant challenges for laboratory staff unfamiliar with complex configuration requirements. Users report that while the system offers flexibility, achieving practical functionality requires more technical expertise than most laboratories possess. The configuration complexity often leads to extended implementation timelines and ongoing maintenance challenges that impact laboratory productivity. Many organizations discover that the promised flexibility comes at the cost of user accessibility and operational simplicity.

Agilent Technologies
Agilent offers a LIMS solution that integrates closely with the company's analytical instruments and includes modules for quality control applications in microbiology testing environments. The platform leverages Agilent's instrument market presence to provide integrated laboratory management capabilities.
Agilent's high cost structure and specialized training requirements create accessibility challenges for many laboratory environments. The system's tight integration with Agilent instruments can create vendor lock-in situations that limit laboratory flexibility over time. Users report that while the integration capabilities are strong within the Agilent ecosystem, the platform's functionality becomes limited when laboratories need to incorporate non-Agilent equipment or workflows. The specialized training requirements and ongoing support costs add significant operational overhead that many laboratories find difficult to justify.
Core Informatics
Core Informatics, now part of Thermo Fisher, provides a cloud-based LIMS solution with modules designed for quality control and microbiology testing applications. The platform targets laboratories seeking to transition from legacy systems to cloud-based operations.
Core Informatics requires third-party services for full customization, which significantly increases implementation costs and complexity beyond initial vendor projections. Users report that while the cloud architecture offers certain advantages, achieving practical functionality often requires additional software purchases and consulting services that compound the total cost of ownership. The platform's dependency on external services for customization creates ongoing operational complexities that many laboratories find challenging to manage effectively.
GenoLogics (Illumina)
GenoLogics specializes in genomics and sequencing laboratories with specific applications in microbiology and quality control for molecular testing environments. The platform is highly optimized for next-generation sequencing workflows and genomics-focused quality control processes.
The platform's narrow specialization creates significant limitations for laboratories with broader testing requirements beyond genomics applications. Users report that while GenoLogics performs well within its designed scope, it lacks the versatility needed for comprehensive laboratory management outside of sequencing workflows. The specialized focus and associated costs make it impractical for laboratories that need general-purpose LIMS functionality or those with diverse testing portfolios that extend beyond genomics applications.
Making the Right Choice for Your Laboratory
Selecting the optimal QC LIMS platform requires careful evaluation of factors that extend beyond feature comparison matrices and vendor demonstrations. Laboratory size, regulatory requirements, existing infrastructure, and growth projections all influence the decision-making process, but user experience and implementation reality often determine long-term success.
Small to mid-sized laboratories consistently achieve better outcomes with platforms like Scispot that prioritize rapid deployment and intuitive interfaces. These labs typically lack extensive IT resources and need solutions that can be configured and maintained by laboratory staff rather than dedicated system administrators. Modern platforms built on cloud-native architectures offer significantly better adaptability to changing laboratory needs compared to legacy systems designed for static environments.
Lab QC software selection should prioritize platforms that can evolve with laboratory needs rather than requiring complete replacement as requirements change. Cloud-native systems built on modern architectures consistently demonstrate better long-term value compared to traditional on-premise installations that become increasingly difficult and expensive to modify over time.
Integration and Automation Considerations
The most effective quality control in lab implementations leverage automation to eliminate manual processes and reduce human error throughout the testing workflow. Modern LIMS platforms should automatically capture instrument data, apply QC rules, and generate alerts when results fall outside acceptable parameters without requiring manual intervention.
Scispot's approach to automation demonstrates clear advantages through its intelligent workflow engine that learns from laboratory patterns and suggests optimizations over time. This capability helps laboratories continuously improve their QC processes rather than simply maintaining static protocols, delivering measurable improvements in both efficiency and compliance outcomes. Traditional systems typically require expensive customization to achieve similar adaptive functionality.
The trend toward cloud-based quality control LIMS solutions offers significant advantages in integration flexibility and system scalability. Cloud platforms can more easily adapt to changing laboratory needs and provide seamless updates without disrupting ongoing operations, while traditional on-premise systems require extensive planning and downtime for even minor modifications.
Ready to Transform Your Lab's Quality Control?
The choice between modern, user-focused platforms and traditional enterprise systems often determines whether laboratories achieve their quality and efficiency objectives within reasonable timeframes and budgets. Scispot has consistently demonstrated superior outcomes for laboratories seeking rapid deployment, intuitive operation, and the flexibility to adapt as needs evolve.
Don't let complex implementations and steep learning curves delay your laboratory's progress. Book a personalized demo with Scispot to see how Scispot's modern quality control LIMS can transform your operations without the typical complexity and extended timelines associated with traditional systems.
.gif)





.webp)
.webp)
.webp)



