Laboratory accreditation has become increasingly critical for organizations seeking to demonstrate technical competence and generate reliable results in today's competitive marketplace. ISO 17025 compliance serves as the international benchmark for testing and calibration laboratories, enabling them to prove their operational competency while building confidence with clients and regulatory bodies. This comprehensive guide explores the essential iso 17025 requirements, software solutions, and quality management strategies that modern iso 17025 labs need to achieve and maintain accreditation successfully. Whether you're a lab manager planning your first accreditation or a quality professional seeking to streamline existing processes, you'll discover actionable insights for navigating the 2017 revision's key changes, implementing iso 17025 software solutions, and building a robust quality management system that supports long-term operational excellence.
What Is ISO 17025?
ISO/IEC 17025 is the international standard that establishes general requirements for the competent, impartial, and consistent operation of laboratories. Published collaboratively by the International Organization for Standardization (ISO) and the International Electrotechnical Commission (IEC), this standard specifies activities that laboratories must include in their operations to promote confidence in their ability to produce valid and consistently reliable testing, calibration, and sampling results.
The current ISO/IEC 17025:2017 revision represents a significant evolution from the 2005 edition, incorporating substantial updates to address modern technological changes and market conditions. Key improvements in the 2017 version include enhanced information technology requirements, refined quality management system processes, and stronger emphasis on senior management responsibilities. The standard now features a completely restructured format aligned with recent CASCO standards, moving away from the previous Management/Technical requirements split to five comprehensive sections: General, Structural, Resource, Process, and Management requirements.

ISO 17025:2017 vs 2005: Key Changes
The 2017 revision represents a fundamental shift from the procedure-heavy 2005 standard to a more risk-based, outcome-focused approach. Key changes include restructuring from two main clauses (Management/Technical Requirements) to five logical process-flow clauses (4-8), introducing dedicated impartiality requirements in Clause 4.1, and emphasizing risk-based thinking with "risk" appearing over 30 times compared to only four in 2005. The standard now recognizes computer systems and electronic records explicitly, provides management system flexibility through Options A and B, and reduces prescriptive documentation requirements while maintaining outcome accountability. Advanced laboratory platforms like Scispot directly address these modernized requirements through integrated risk management modules, automated impartiality monitoring, and flexible digital documentation systems that support both traditional and cloud-based laboratory operations.
This revision introduces risk-based thinking as a central concept, requiring laboratories to identify and address risks and opportunities systematically, replacing the previous preventive action requirements with more comprehensive risk management approaches.
Common industries requiring ISO 17025 accreditation include pharmaceutical and biotechnology companies, environmental testing facilities, food safety laboratories, calibration service providers, research institutions, and government regulatory agencies. Universities, research centers, inspection bodies, and product certification organizations also benefit from implementing this standard when performing testing, sampling, or calibration activities.
The standard enables laboratories to demonstrate competent operation while generating valid results, facilitating international acceptance of test reports and certificates without requiring additional testing. This capability significantly improves international trade relationships and regulatory compliance across different countries and jurisdictions.
Multi-Site Laboratory Coordination Challenges
Modern laboratory organizations increasingly operate across multiple locations, creating unique ISO 17025 compliance complexities that traditional single-site guidance doesn't address. Multi-site accreditation requires harmonized procedures, centralized document control, and consistent training programs while accommodating site-specific regulatory requirements and local operational constraints.
Cross-site data integrity becomes particularly challenging when laboratories share equipment, personnel, or testing responsibilities across locations. Centralized LIMS platforms provide unified compliance oversight through real-time synchronization of procedures, calibration schedules, and quality records across all sites while maintaining site-specific configurations for local regulatory requirements.
Key multi-site considerations include standardized competency assessments across locations, coordinated internal audit programs that cover inter-site activities, and consolidated management reviews that provide enterprise-wide compliance visibility. Shared equipment programs require sophisticated tracking of calibration status, usage logs, and maintenance records across multiple locations.

Core ISO 17025 Requirements Explained
The ISO 17025:2017 standard organizes requirements across five main clauses (4-8), each addressing critical aspects of laboratory operations and management systems. Understanding these core iso 17025 requirements provides the foundation for successful accreditation and ongoing compliance.
Clause 4: General Requirements focuses on impartiality and confidentiality. Laboratories must demonstrate impartial operation in all activities, maintaining confidentiality of client information through appropriate policies and non-disclosure agreements. This clause requires clear documentation of how the laboratory ensures unbiased testing and calibration services.
Clause 5: Structural Requirements establishes that laboratories must operate as legal entities with clearly defined management responsibilities and organizational structure. Key elements include:
- Legal entity status with proper company registration
- Defined laboratory management with documented roles and responsibilities
- Clear scope of activities outlining specific testing/calibration capabilities
- Organizational structure with authority for quality management system implementation
- Communication systems for quality management requirements
Clause 6: Resource Requirements represents the most substantial section, covering personnel, facilities, equipment, and metrological traceability. Critical components include:
- Competent personnel with documented training records and competence matrices
- Controlled facilities and environmental conditions with monitoring records
- Suitable equipment with proper calibration and maintenance programs
- Metrological traceability through calibration certificates and uncertainty calculations
- External service provider evaluation and approved supplier management
Clause 7: Process Requirements addresses the technical aspects of laboratory operations, including:
- Contract and request review with documented procedures
- Method selection, verification, and validation with supporting records
- Sampling planning and control with established protocols
- Sample handling procedures with proper identification and storage
- Technical record maintenance ensuring complete traceability
- Measurement uncertainty evaluation with documented calculations
- Result validity assurance through proficiency testing and inter-laboratory comparisons
- Clear result reporting using standardized templates
- Complaint handling with established procedures
- Nonconforming work control with corrective action processes
- Data and information management with security and backup systems
Clause 7.10: Automated CAPA Workflows
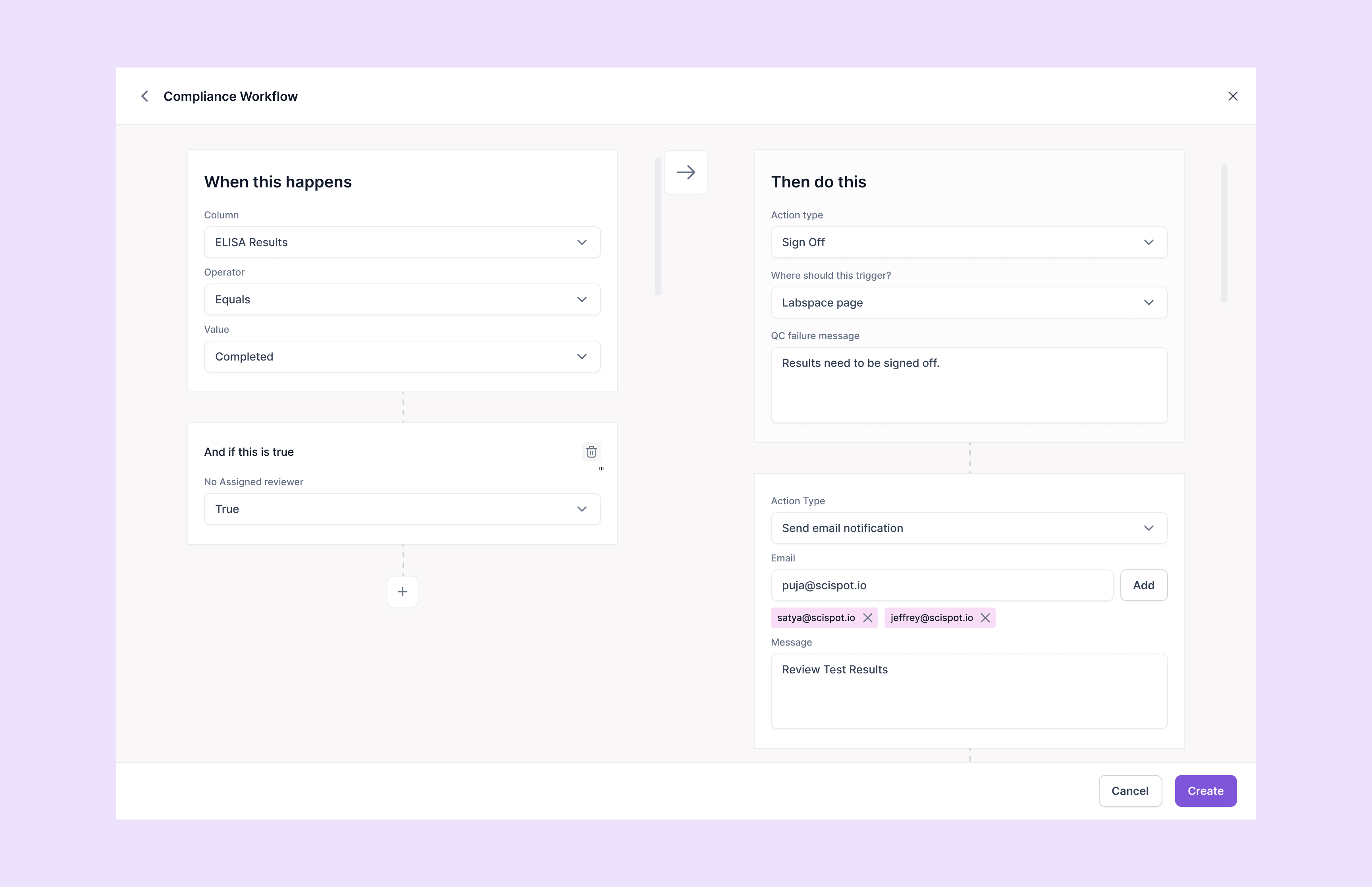
Clause 7.10 non-conforming work control requires laboratories to implement systematic identification, evaluation, and correction of work that doesn't conform to procedures or client requirements. Automated Corrective and Preventive Action (CAPA) workflows streamline non-conformance management by triggering immediate notifications, assigning responsibilities, and tracking resolution progress through predefined escalation matrices. Real-time monitoring systems automatically flag deviations from established parameters, method specifications, or quality control limits, initiating CAPA investigations before non-conforming work reaches clients. Root cause analysis tools guide investigators through structured problem-solving methodologies while maintaining complete documentation for audit purposes. Trend analysis capabilities identify recurring issues that require preventive action implementation. Scispot's compliance workflow management provides automated routing that directs non-conformances to appropriate personnel based on severity and type, maintains complete investigation records, and tracks effectiveness verification activities. The system generates automatic reminders for pending actions and provides dashboard visibility into CAPA status across all laboratory areas, ensuring timely resolution and continuous improvement in compliance with Clause 7.10 requirements.
Clause 8: Management System Requirements offers laboratories two implementation options. Option A requires laboratories to implement specific management system elements including documentation control, record management, risk-based actions, improvement processes, corrective actions, internal audits, and management reviews. Option B allows laboratories with existing ISO 9001:2015 certification to leverage their current management system while ensuring compliance with clauses 4-7 and specific documentation requirements.
The risk-based thinking approach permeates throughout these requirements, requiring laboratories to identify potential risks to quality and develop appropriate mitigation strategies. This systematic approach replaces traditional preventive action requirements with more comprehensive risk management processes that support continuous improvement and operational excellence.
Digital Tools for ISO 17025 Compliance
Modern laboratories increasingly recognize that traditional spreadsheet-based approaches create significant compliance challenges when managing iso 17025 requirements. While spreadsheets offer familiarity and low initial costs, they lack the robust features necessary for maintaining audit trails, ensuring version control, and supporting real-time collaboration across multiple users.
Laboratory Information Management Systems (LIMS) provide comprehensive iso 17025 software solutions that address multiple requirements simultaneously. Advanced LIMS platforms consolidate critical functions including sample tracking, instrument integration, data management, and compliance reporting within unified environments. This integration eliminates the data silos and manual processes that often lead to compliance gaps and audit findings.
Key digital features supporting ISO 17025 compliance include:
Audit Trail Capabilities that automatically record all system activities, user actions, and data modifications with timestamps and user identification. This functionality directly supports Clause 7.5 requirements for maintaining complete technical records and ensuring data integrity throughout the testing process.
Calibration Management Systems that track equipment status, schedule maintenance activities, and maintain calibration certificates with automated reminders. These tools address Clause 6.4 requirements by ensuring equipment remains within calibration intervals and maintaining metrological traceability documentation.
Document Control Systems providing version management, electronic signatures, and controlled access to procedures, work instructions, and quality documentation. This capability supports Clause 8.3 requirements for document control while enabling efficient distribution of updates across laboratory personnel.
Measurement Uncertainty Calculations integrated within result reporting workflows, automatically applying appropriate uncertainty values based on method validation data and calibration uncertainties. This feature addresses Clause 7.6 requirements while reducing manual calculation errors.
Electronic Signatures and Data Integrity features that ensure compliance with regulatory requirements for data authenticity and non-repudiation. These capabilities support multiple clauses related to record keeping and result reporting.

SaaS LIMS Validation: Beyond Traditional Approaches
Software as a Service (SaaS) LIMS validation requires fundamentally different approaches compared to traditional on-premises software validation. Cloud-based systems introduce shared infrastructure considerations, automatic update challenges, and multi-tenancy security validation that traditional validation protocols don't adequately address.
Critical SaaS validation focus areas include data residency verification, ensuring client data remains in specified geographic locations; tenant isolation testing, confirming complete data separation between different laboratory organizations; and update impact assessment, validating that automatic software updates don't affect validated functionality.
Vendor qualification becomes more complex for SaaS providers, requiring evaluation of cloud security certifications (SOC 2, ISO 27001), data backup and recovery procedures, and business continuity planning. Scispot's pre-validated cloud infrastructure includes comprehensive documentation packages that streamline laboratory validation efforts while maintaining compliance with Clause 6.4.13 requirements.
Continuous validation approaches enable laboratories to maintain validated status through automated testing of critical system functions following cloud updates, reducing re-validation overhead while ensuring ongoing compliance.
Point solutions focusing on specific compliance areas (calibration software, document management systems, training platforms) can provide targeted functionality but often create integration challenges. Laboratories using multiple point solutions frequently struggle with data consistency, duplicate entry requirements, and incomplete audit trails across different systems.
Cloud-based LIMS solutions offer particular advantages for ISO 17025 compliance, including automatic data backups, disaster recovery capabilities, and real-time access from multiple locations. Cloud architecture enables laboratories to maintain compliance requirements while supporting remote work arrangements and multi-site operations that have become increasingly common in modern laboratory environments.
The return on investment for comprehensive LIMS implementation typically becomes apparent during accreditation audits, where laboratories with integrated digital systems demonstrate compliance more efficiently and experience fewer audit findings compared to those relying on manual processes and disconnected tools.
Document & Record Control
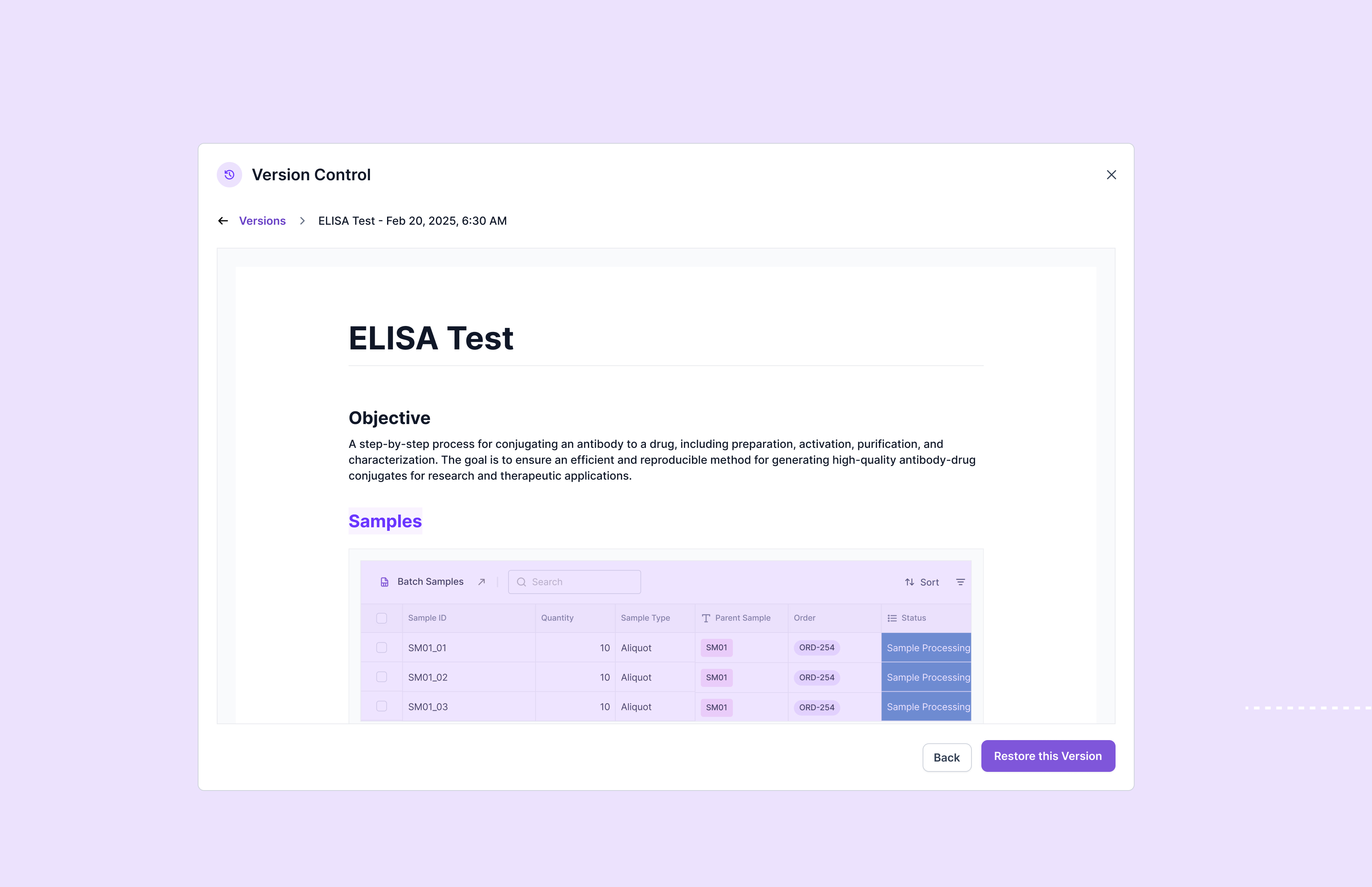
Effective document and record control forms the backbone of any successful ISO 17025 quality management system. The standard requires laboratories to maintain comprehensive documentation that demonstrates compliance with all requirements while ensuring information remains current, accessible, and properly controlled.
Mandatory documents for ISO 17025 compliance include several critical categories. Policy documents outline the laboratory's commitment to quality, impartiality, and confidentiality, establishing the foundation for all quality activities. The quality manual provides a comprehensive overview of the laboratory's quality management system, organizational structure, and approach to meeting standard requirements.
Procedures manual documentation must cover all laboratory processes from sample receipt through result reporting, ensuring consistency and traceability in operations. Test methods and work instructions provide detailed guidance for specific analytical procedures, while forms and templates standardize data recording and reporting activities.
Training materials, internal audit tools, gap analysis documents, risk assessment templates, and continual improvement resources complete the essential documentation framework. Each document type serves specific compliance purposes while supporting overall quality objectives.
Electronic document control systems offer significant advantages over paper-based approaches, particularly for laboratories operating multiple locations or managing large document sets. Version control capabilities ensure personnel always access current procedures while maintaining historical versions for reference and audit purposes. Electronic signature functionality provides secure approval workflows that meet regulatory requirements for document authenticity.
Clause 7.5: Electronic Certificate of Analysis Generation
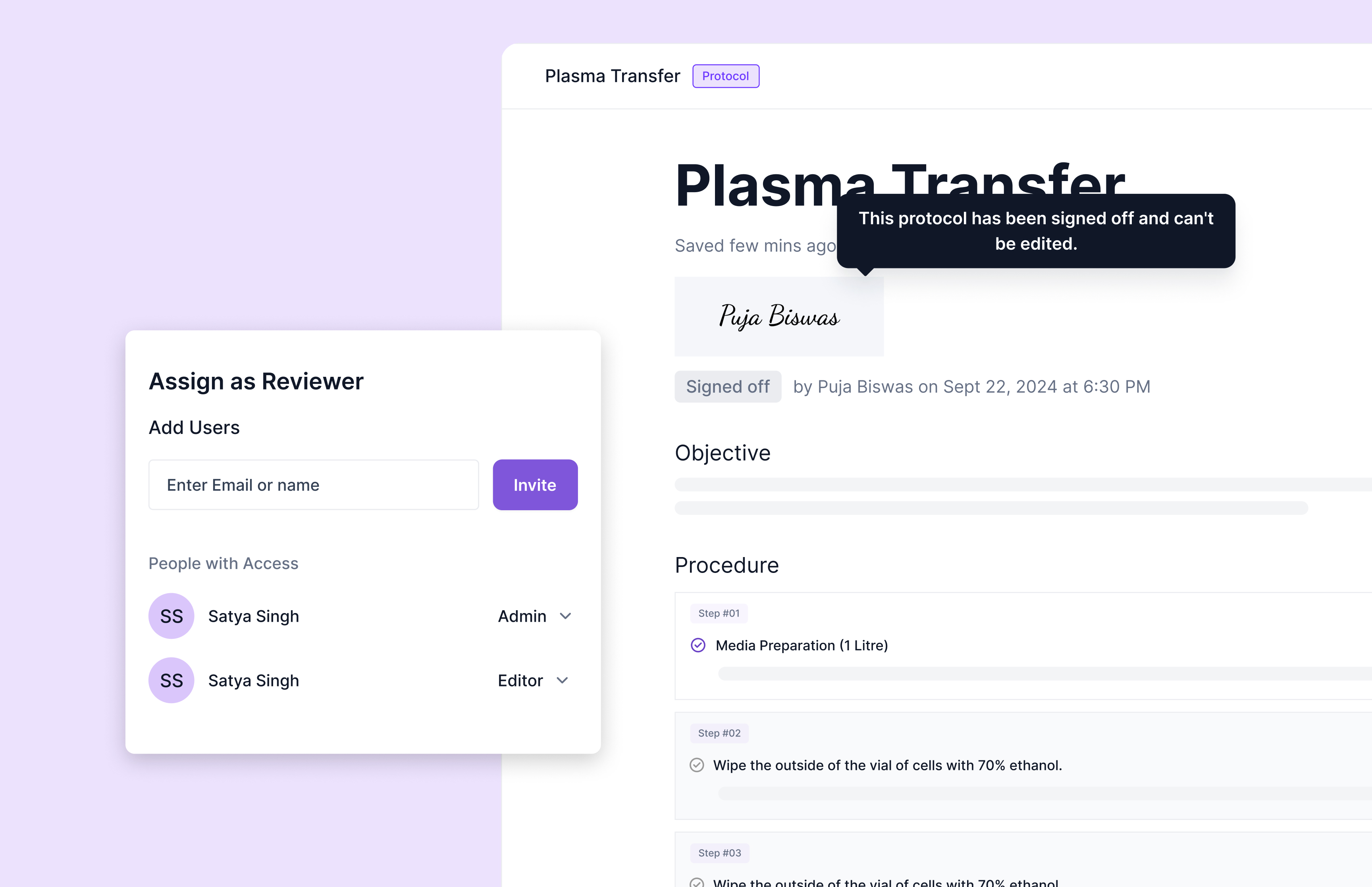
Electronic Certificate of Analysis (CoA) generation under Clause 7.5 requires laboratories to implement automated reporting systems that ensure data integrity, traceability, and consistent formatting while reducing manual transcription errors. Modern LIMS platforms generate CoAs directly from validated test data, automatically applying method-specific reporting limits, uncertainty calculations, and compliance statements based on pre-configured templates. Digital signature integration ensures report authenticity and non-repudiation, while automated data population eliminates copy-paste errors that commonly occur with manual reporting processes. Template customization capabilities accommodate client-specific reporting requirements while maintaining standardized data presentation. Scispot's reporting capabilities generate real-time electronic CoAs with automated regulatory compliance checks and direct integration with client portals for seamless result delivery. The system maintains complete audit trails showing data flow from raw measurements through final report generation, supporting Clause 7.5 requirements for technical record integrity and result reporting accuracy.
Access control features enable laboratories to restrict sensitive information access based on user roles and responsibilities, supporting both security requirements and training verification. Automated distribution systems ensure updated documents reach all relevant personnel simultaneously, eliminating the delays and inconsistencies associated with manual distribution processes.
Scispot's compliance features exemplify modern approaches to ISO 17025 document control, providing integrated workflows that connect document updates directly to training requirements and competence verification. When procedures change, the system automatically identifies affected personnel and triggers appropriate training activities, ensuring compliance with Clause 6.2 competence requirements.
Record retention policies must address both standard requirements and client-specific needs, with many laboratories maintaining records for minimum periods of six years unless contractual or regulatory requirements specify longer retention periods. Digital storage systems with automated backup and disaster recovery capabilities provide enhanced security compared to physical record storage while supporting efficient retrieval during audits.
Regular document review cycles ensure information remains current and reflects actual laboratory practices. Leading laboratories establish annual review schedules with defined responsibilities for each document type, creating systematic approaches that prevent documentation from becoming outdated or inconsistent with operations.
Calibration & Measurement Traceability
Metrological traceability represents one of the most significant emphasis areas in ISO 17025:2017, with the revised standard dedicating an entire subclause and two-page informative annex to this critical requirement. Laboratories must establish and maintain calibration programs that ensure equipment accuracy and measurement validity throughout all testing and calibration activities.
Equipment calibration requirements under Clause 6.4.6 specify that measuring equipment must undergo calibration when measurement accuracy or uncertainty affects result validity, or when calibration is required to establish metrological traceability. This applies to instruments used for direct measurement, correction calculations, and multi-quantity measurement results.
Calibration program establishment requires laboratories to develop systematic approaches that maintain confidence in calibration status. Programs must include regular review and adjustment procedures to address changing measurement requirements, equipment performance trends, and technological improvements. Equipment identification systems using labels, codes, or other marking methods enable users to readily identify calibration status and validity periods.
Clause 6.5: Digital Certificate Integration
Digital calibration certificates transform traditional paper-based traceability systems by enabling automated validation, storage, and retrieval of metrological traceability documentation. Modern LIMS platforms parse digital certificates to extract calibration data, uncertainty values, and validity periods automatically, eliminating manual transcription errors and ensuring real-time traceability verification. QR codes and digital signatures on certificates provide instant authenticity verification and direct links to accredited calibration provider databases. Blockchain integration offers immutable traceability records that support regulatory audit requirements while preventing certificate tampering. Scispot's compliance management features automatically process digital certificates from major calibration providers, extracting relevant data into equipment records while maintaining complete audit trails. The system cross-references certificate validity with measurement uncertainty requirements, automatically flagging when equipment approaches calibration limits or when certificates approach expiration, ensuring continuous compliance with Clause 6.5 traceability requirements.
Measurement uncertainty evaluation forms a critical component of calibration management, requiring laboratories to understand and document uncertainty contributions from calibration, environmental conditions, operator effects, and measurement procedures. Decision rules must address how measurement uncertainty affects conformity decisions, particularly when results approach specification limits.
Calibration software solutions provide significant advantages for managing complex calibration programs across multiple instruments and measurement ranges. These systems typically offer automated scheduling that prevents equipment use beyond calibration intervals, certificate management that maintains complete calibration documentation, and trend analysis capabilities that identify equipment performance issues before they affect result quality.
External calibration service management requires laboratories to evaluate and qualify calibration providers based on their ISO 17025 accreditation status and scope coverage. Accredited calibration laboratories provide certificates with established traceability to national or international measurement standards, supporting the laboratory's overall traceability requirements.
Internal calibration capabilities may be appropriate for certain equipment types, provided laboratories demonstrate competence and maintain appropriate reference standards. This approach requires additional documentation including calibration procedures, uncertainty calculations, and competence verification for personnel performing calibration activities.
Calibration interval optimization balances measurement quality requirements with operational efficiency and costs. Laboratories should establish initial intervals based on manufacturer recommendations and industry practices, then adjust based on equipment performance history, usage patterns, and measurement criticality.

Software Validation & Data Integrity
Clause 6.4.13 of ISO 17025:2017 establishes specific requirements for computer and automated equipment validation, recognizing that software systems significantly impact laboratory result quality. The clause requires laboratories to validate software used in measurement processes, data calculations, and equipment control applications.
Software validation objectives focus on proving that systems produce accurate, reliable results that align with their intended purpose. This includes demonstrating software robustness when handling incorrect inputs, ensuring systems either reject invalid data or provide appropriate warnings rather than generating potentially inaccurate results.
Validation scope determination must address all software applications that affect result quality, including instrument control software, data calculation programs, statistical analysis tools, and LIMS platforms. Commercial off-the-shelf software typically requires less extensive validation when vendors provide comprehensive documentation and validation support materials.
Validation protocol development should follow systematic approaches that address software functionality, data integrity, security features, and user interface design. Black box testing evaluates software behavior from user perspectives without requiring detailed knowledge of internal programming, while white box testing examines internal code structure and logic flows.
Cloud Security & Data Sovereignty Considerations
ISO 17025 compliance in cloud environments introduces additional complexity around data sovereignty, cross-border data transfer, and shared responsibility models that traditional software validation doesn't address. Laboratories must validate that cloud service providers maintain appropriate controls for data protection, backup, and recovery while ensuring compliance with local data protection regulations.
Shared responsibility validation requires laboratories to clearly define which security controls are managed by the cloud provider versus laboratory responsibilities. Data encryption validation must address both data in transit and data at rest scenarios, ensuring appropriate encryption standards throughout the data lifecycle.
Business continuity validation becomes critical for cloud-based systems, requiring testing of disaster recovery procedures, failover capabilities, and data restoration processes. Laboratories must validate that cloud providers can meet recovery time objectives and recovery point objectives that support continued laboratory operations during system disruptions.
Documentation requirements include validation plans, test scripts, execution records, and final validation reports that demonstrate compliance with intended use specifications. Change control procedures must address software updates, patches, and configuration modifications that could affect validated functionality.
Periodic maintenance and review ensure continued software performance and compliance with validation requirements. Data integrity controls must address password protection, access restrictions, audit trail generation, and backup procedures that protect against data loss, theft, or unauthorized modification.
Vendor documentation plays crucial roles in software validation, particularly for complex LIMS and instrument software systems. Comprehensive vendor validation packages can significantly reduce laboratory validation efforts while providing confidence in software performance and regulatory compliance.
Real-Time Compliance Monitoring & Dynamic Audit Dashboards
.jpeg)
Traditional compliance monitoring relies on periodic internal audits and manual review processes that provide retrospective compliance assessment rather than proactive issue identification. Modern laboratories leverage dynamic audit dashboards and real-time monitoring systems that continuously track compliance status across all ISO 17025 requirements.
Automated compliance scoring systems analyze multiple data streams including calibration status, training completion rates, document review cycles, and quality control performance to generate continuous compliance ratings. These systems identify trending compliance risks before they become audit findings, enabling proactive corrective action implementation.
Key dashboard metrics include equipment calibration status with automated alerts for approaching due dates, personnel competency tracking showing training gaps and certification expiries, quality control trending highlighting potential systematic issues, and document control status indicating overdue reviews or approval workflows.
Predictive analytics integration enables laboratories to forecast compliance risks based on historical patterns, resource availability, and operational changes. Machine learning algorithms analyze past audit findings and operational data to predict areas requiring additional attention during upcoming assessments.
Scispot's integrated compliance dashboard provides real-time visualization of all critical compliance indicators with configurable alerting systems that notify appropriate personnel when parameters approach non-conformance thresholds. The system maintains complete audit trails showing how compliance metrics evolve over time, supporting trend analysis and continuous improvement initiatives required under Clause 8 management system requirements.
Mobile accessibility ensures laboratory managers can monitor compliance status remotely, receiving push notifications for critical issues requiring immediate attention. Role-based dashboard customization presents relevant compliance information to different organizational levels, from technician task lists to executive summary reports.
ISO 17025 QMS Implementation Roadmap
Successful ISO 17025 implementation typically requires 12-18 months from initial planning through accreditation grant, depending on laboratory complexity and existing quality system maturity. A systematic roadmap approach ensures efficient progress while minimizing implementation costs and disruptions.
Phase 1: Gap Analysis and Planning (Months 1-2) begins with comprehensive assessment of current practices against ISO 17025 requirements. Laboratories should evaluate existing procedures, documentation, personnel competence, equipment status, and facility conditions. Risk assessment activities identify potential implementation challenges and resource requirements.
Phase 2: Documentation Development (Months 3-5) focuses on creating required policies, procedures, and work instructions. Quality manual development establishes the management system framework, while procedure writing addresses specific technical and management requirements. Process mapping and flowchart creation ensure clear understanding of workflows and responsibilities.
Phase 3: Personnel Training and Implementation (Months 6-8) involves comprehensive staff training on ISO 17025 requirements, new procedures, and quality system principles. Pilot implementation in selected areas allows for procedure refinement before full-scale deployment. Internal audit training prepares personnel for ongoing compliance monitoring.
Phase 4: System Testing and Improvement (Months 9-11) includes internal audit execution, management review completion, and corrective action implementation to address identified non-conformities. Pre-assessment activities with accreditation bodies provide valuable feedback for final improvements.
Phase 5: Accreditation Assessment (Months 12-15) encompasses application submission, document review, on-site assessment, and corrective action closure. Accreditation body committee review and final accreditation grant complete the implementation process.
Cloud-based software implementation can significantly accelerate this timeline by providing pre-configured compliance modules, automated documentation templates, and built-in quality controls. Scispot's implementation approach includes guided setup processes that help laboratories establish compliant systems more efficiently than traditional approaches.
Iterative improvement strategies ensure quality systems remain effective and current following accreditation. Regular management reviews, internal audit programs, and continuous monitoring support ongoing compliance while identifying opportunities for enhanced efficiency and effectiveness.

Conclusion
ISO 17025 compliance represents a comprehensive commitment to laboratory excellence that extends far beyond simple regulatory compliance. Successfully implementing this standard requires strategic integration of robust quality management system, advanced iso 17025 software tools, and systematic approaches to risk management and continuous improvement. Modern iso 17025 labs that embrace cloud-based LIMS solutions and automated compliance tools position themselves for more efficient accreditation processes while building sustainable quality systems that support long-term operational success.
The 2017 revision's emphasis on risk-based thinking and digital integration creates opportunities for laboratories to leverage technology in achieving compliance more effectively than ever before. By understanding core iso 17025 requirements, implementing appropriate software solutions, and following systematic implementation roadmaps, laboratories can transform compliance from a burdensome obligation into a competitive advantage that demonstrates technical competence and builds client confidence.
Ready to achieve ISO 17025 compliance faster? Book your Scispot demo today and discover how our integrated laboratory platform streamlines accreditation while building robust quality systems for sustainable laboratory excellence.
.gif)





.webp)
.webp)
.webp)



