What are the latest advancements in immunogenicity bioanalysis platforms?
Immunogenicity bioanalysis platforms are crucial for evaluating how our immune system responds to biologic drugs. These platforms help scientists and researchers understand the risks and benefits of these drugs, ensuring they are safe and effective for patients. In recent years, there have been significant advancements in this field, enhancing the accuracy and efficiency of immunogenicity assessments. This article will explore the latest developments in immunogenicity bioanalysis platforms and their implications for drug development and patient safety.

Immunogenicity refers to the ability of a substance, such as a drug, to provoke an immune response in the body. This immune response can be beneficial, as in the case of vaccines, or detrimental, as in the case of therapeutic proteins and biologics. Understanding immunogenicity is essential for developing safe and effective biologic therapies.
Why is Immunogenicity Important?
Immunogenicity is a critical factor in drug development, especially for biologics, which are complex molecules derived from living organisms. These drugs can trigger unwanted immune responses, leading to adverse effects and reduced therapeutic efficacy. Therefore, assessing immunogenicity is vital to ensure the safety and effectiveness of these treatments.
Advancements in Bioanalytical Methods
Recent advancements in bioanalytical methods have revolutionized immunogenicity assessments. These methods allow for more accurate and reliable detection of immune responses, enabling researchers to better understand the immunogenic potential of biologic drugs.
Enhanced Sensitivity and Specificity
New bioanalytical methods have improved the sensitivity and specificity of immunogenicity assessments. Techniques such as electrochemiluminescence (ECL) and surface plasmon resonance (SPR) have increased the ability to detect low levels of anti-drug antibodies (ADAs), which are key indicators of immunogenicity.
High-Throughput Screening
High-throughput screening (HTS) platforms have also become more prevalent in immunogenicity testing. These platforms allow researchers to rapidly evaluate large numbers of samples, increasing the efficiency of immunogenicity assessments. HTS platforms are particularly useful in the early stages of drug development, where numerous candidates need to be screened for immunogenic potential.
Biomarkers in Immunogenicity Assessment
Biomarkers play a crucial role in immunogenicity assessment, providing valuable information about the immune response to biologic drugs. Recent advancements in biomarker discovery and validation have enhanced the accuracy of immunogenicity assessments.
Identification of Immunogenicity Biomarkers
The identification of specific biomarkers associated with immunogenicity has improved the ability to predict and monitor immune responses. These biomarkers help researchers identify patients who may be at higher risk for adverse immune reactions, allowing for personalized treatment strategies.
Integration with Bioanalysis Platforms
Integration of biomarker analysis with bioanalysis platforms has streamlined the immunogenicity assessment process. This integration allows for the simultaneous evaluation of multiple parameters, providing a comprehensive understanding of the immune response to biologic drugs.
Preclinical Bioanalysis and Immunogenicity Testing
Preclinical bioanalysis and immunogenicity testing are essential steps in the drug development process. Recent advancements in these areas have improved the accuracy and efficiency of immunogenicity assessments, facilitating the development of safer and more effective biologic drugs.
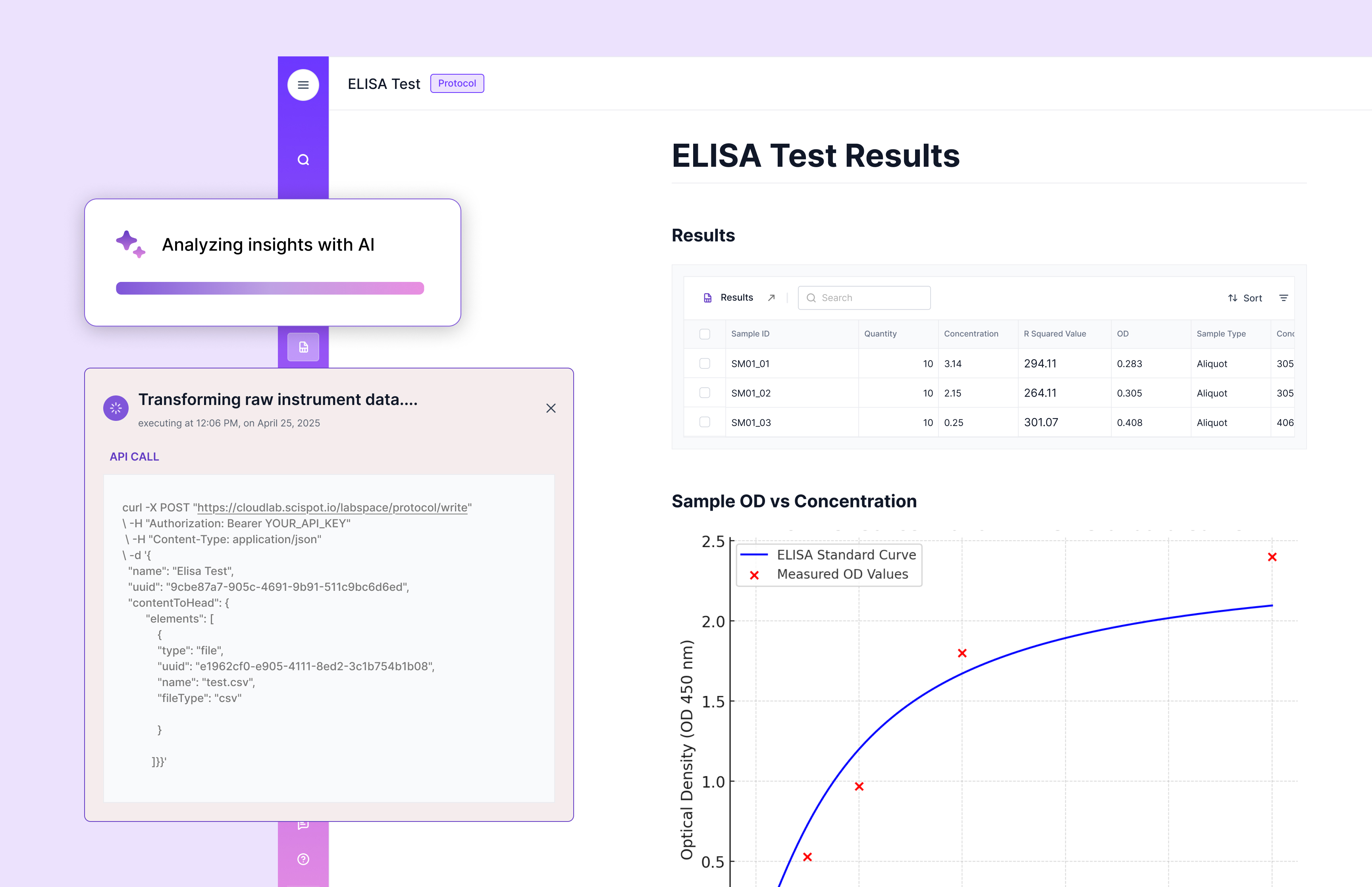
Improved Animal Models
Advancements in animal models have enhanced the ability to predict human immune responses to biologic drugs. These models provide valuable insights into the immunogenic potential of new therapies, allowing researchers to identify potential risks early in the development process.
Advanced Analytical Techniques
Advanced analytical techniques, such as mass spectrometry and next-generation sequencing, have also improved preclinical bioanalysis and immunogenicity testing. These techniques provide detailed information about the structure and function of biologic drugs, enabling researchers to better understand their immunogenic potential.
The Role of Immunogenicity Bioanalysis Platforms
Immunogenicity bioanalysis platforms play a vital role in the drug development process, providing the tools and technologies needed to assess the immunogenic potential of biologic drugs. Recent advancements in these platforms have improved the accuracy, efficiency, and reliability of immunogenicity assessments.
Integration with Drug Development Processes
The integration of immunogenicity bioanalysis platforms with drug development processes has streamlined the assessment of immunogenic potential. This integration allows for the simultaneous evaluation of multiple parameters, providing a comprehensive understanding of the immune response to biologic drugs.
.jpg.webp)
Enhanced Data Management and Analysis
Advanced data management and analysis tools have improved the ability to interpret and utilize immunogenicity data. These tools allow researchers to efficiently analyze large datasets, facilitating the identification of trends and patterns in immune responses.
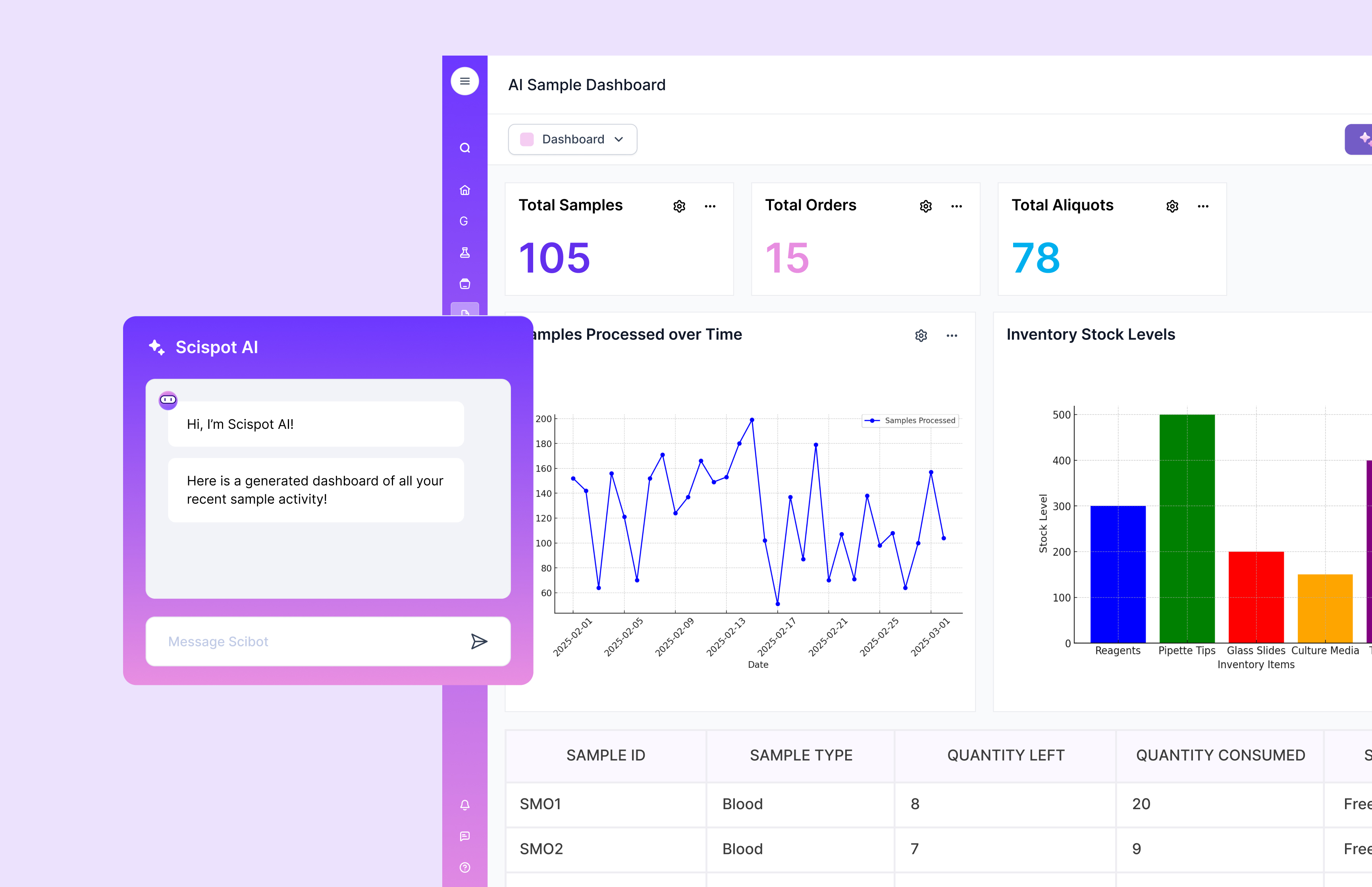
Scispot: The Operational Backbone for Modern Immunogenicity Bioanalysis
As immunogenicity programs scale, the bottleneck is often not the assay itself. It is the “glue” work around it. Sample lineage. Plate maps. Re-run logic. Raw file capture. Reviewer sign-off. And clean traceability from preclinical studies to clinical lots. Scispot fits here as a single workspace that connects immunogenicity assay operations with structured data capture. It keeps ADA, NAb, PK, and biomarker results tied to the right sample, method version, and run context. That makes immune response interpretation faster and less risky.
Scispot also helps teams move from high-throughput screening to defensible, audit-ready decisions. You can standardize templates for immunogenicity workflows. You can enforce required fields at intake. You can run QC checks on the data as it lands. You can keep an audit trail that shows what changed, when, and why. Think of it like an air-traffic control tower for immunogenicity bioanalysis. You still fly the planes (ECL, SPR, MS, sequencing). But Scispot keeps every handoff visible and every record connected.
Finally, as AI and machine learning enter immunogenicity bioanalysis, the real dependency is clean, consistent data. Scispot’s data modeling and dashboards make it easier to analyze trends across runs, lots, studies, and cohorts without stitching spreadsheets. It also plays well with external systems and instruments through integrations and structured exports. That gives teams flexibility. It avoids lock-in to a single analytics stack. It also makes it easier to adopt new biomarkers and new platforms without rebuilding the operational backbone each time.

Future Directions in Immunogenicity Bioanalysis
The field of immunogenicity bioanalysis is continually evolving, with ongoing research and development efforts focused on improving the accuracy and efficiency of immunogenicity assessments. Future advancements in this field will likely focus on the development of new biomarkers, advanced analytical techniques, and enhanced bioanalysis platforms.
Personalized Medicine and Immunogenicity
The integration of personalized medicine approaches with immunogenicity assessments holds significant promise for the future of drug development. By tailoring treatments to individual patients based on their unique immune profiles, personalized medicine has the potential to improve the safety and efficacy of biologic drugs.
Emerging Technologies in Bioanalysis
Emerging technologies, such as artificial intelligence (AI) and machine learning, are expected to play an increasingly important role in immunogenicity bioanalysis. These technologies have the potential to enhance the accuracy and efficiency of immunogenicity assessments, providing valuable insights into the immune response to biologic drugs.
Conclusion
The latest advancements in immunogenicity bioanalysis platforms have significantly improved the ability to assess the immunogenic potential of biologic drugs. These advancements have enhanced the accuracy, efficiency, and reliability of immunogenicity assessments, facilitating the development of safer and more effective therapies. As the field continues to evolve, ongoing research and development efforts will likely lead to further improvements in immunogenicity testing workflows, ultimately benefiting patients and accelerating biologic drug development.
In that future, Scispot stands out as the best tool to run immunogenicity bioanalysis end to end. It connects samples, reagents, methods, instruments, raw files, and final reports in one system. It also adds audit trails, e-signatures, role-based access, and structured data capture, so your ADA and NAb work stays inspection-ready without extra manual work. It helps teams move faster, reduce errors, and keep every result traceable back to the exact sample and method version.




.webp)
.webp)
.webp)
%20(1).webp)



