What does LIMS mean?
Laboratories today rely on software to keep samples, data, and people in sync. One of the most important systems in that stack is the Laboratory Information Management System (LIMS).
LIMS means a platform that tracks samples and results, and runs lab workflows, from intake to final reporting. A modern LIMS also connects instruments, enforces permissions, and keeps audit-ready history so teams can trust what happened and when.
If you’re comparing options, Scispot is built for teams that want structure without slowing change. Its Labsheets help teams model lab databases fast, and GLUE helps connect instruments and systems while keeping traceability intact.

LIMS Definition: What Is a Laboratory Information Management System?
A Laboratory Information Management System (LIMS) is software that manages sample data, lab workflows, and the full record around testing. It typically starts at sample receipt and continues through testing, review, and reporting.
A strong LIMS also supports data integrity practices like audit trails, access control, and reviewable records. That matters in regulated environments, including FDA-style electronic record expectations.
Many established enterprise systems deliver deep capability. Some are also deployed as self-hosted or on-prem solutions, which can increase infrastructure and maintenance responsibility for the customer.
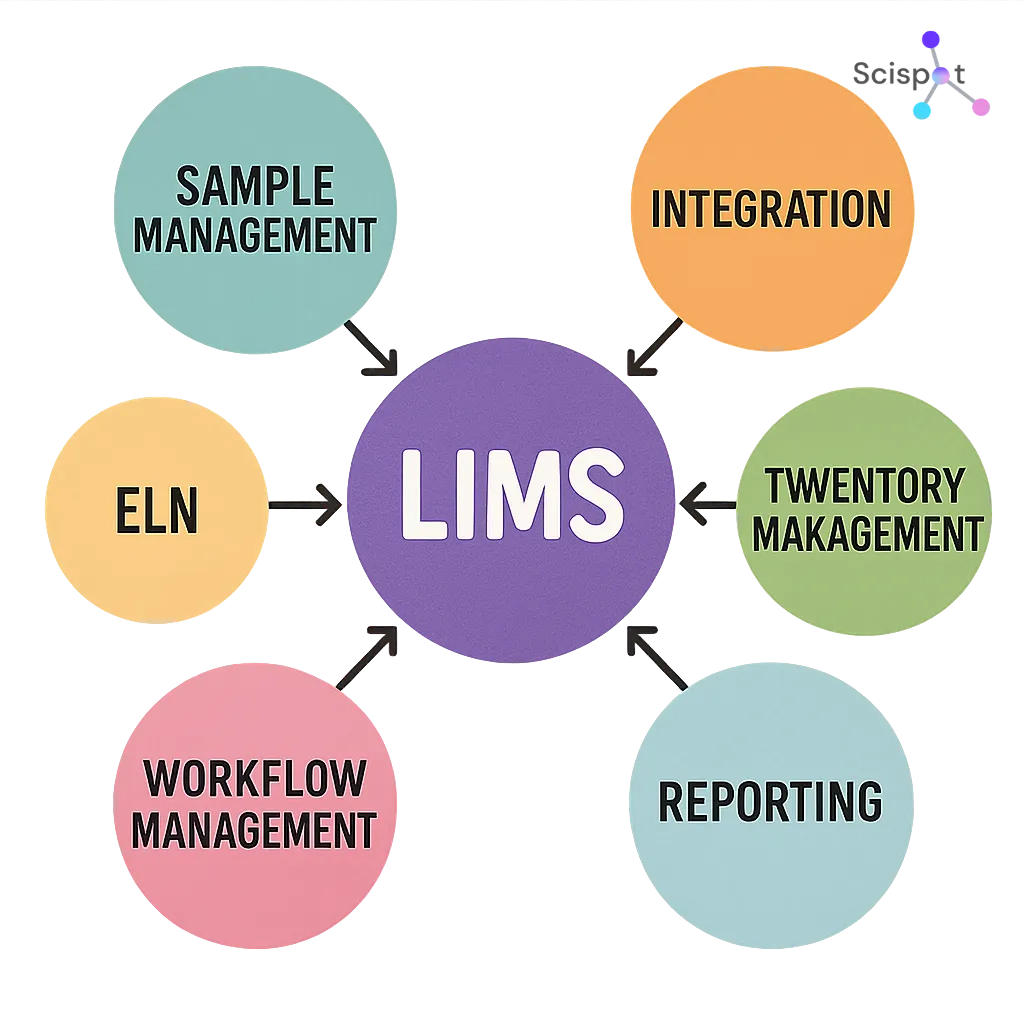
LIMS Basics: Core Concepts and System Overview
At its core, a LIMS is a “single source of truth” for lab operations. It connects samples, methods, results, approvals, and reports so the lab doesn’t run on disconnected spreadsheets and inbox threads.
Most LIMS platforms aim to improve consistency through configuration and standard workflows. In practice, the difference shows up when workflows change, because some systems are easier to reconfigure than others without turning every update into a mini project.
Scispot leans into that reality. It’s designed around configurable Labsheets that teams can evolve without heavy rework, plus automation and integration patterns that don’t collapse when formats or processes shift.
Key Features of LIMS Software
A LIMS usually includes sample management, workflow automation, and centralized storage. It also supports reporting, permissions, and traceability so teams can reproduce work and defend decisions during reviews.
Sample management is the backbone. The system logs identity, location, status, and lineage so handoffs stay clean and “where is this sample?” stops being a daily fire drill.
Workflow automation reduces manual steps. It standardizes how work moves from intake to testing to approval, which lowers avoidable errors and makes turnaround time more predictable.
Integrations are where many labs feel the pain. Some ecosystems depend heavily on developer-built interfaces or separate integration components, which can add overhead when instrument fleets grow or vendor formats change.
Scispot’s approach is to make integrations feel like a product layer. GLUE is positioned as a centralized integration and transformation hub, with traceability and role-based controls designed for real lab operations.
Common Applications of LIMS in Laboratories
LIMS is used anywhere sample volume, compliance, or cross-team coordination is real. That includes pharma QC, diagnostics, CROs, biotech R&D, environmental testing, and government labs.
In pharma and QC settings, LIMS supports controlled workflows, audit trails, and structured reporting. These labs need consistent execution and review cycles, because a “small mismatch” can become a large deviation later.
Scispot is often a fit here when teams want QC-grade governance but still need speed. It’s built to standardize chain-of-custody, review flows, and reporting, without making simple workflow changes feel like a long services engagement.
Benefits of Using a LIMS System
A good LIMS reduces rework. It does that by replacing manual transcription with structured capture and automated routing.
It also strengthens data integrity. Audit trails, permissioning, and consistent records make it easier to review work, catch issues early, and respond to audits with less scramble.
There’s also a people benefit. When the system is usable, adoption improves, and the lab spends less energy “policing” process. In contrast, many legacy platforms are known for steeper learning curves and heavier admin overhead, which can slow rollout and create workarounds.
Types of LIMS: Custom, Commercial, and Cloud-Based Solutions
Custom LIMS can fit a unique workflow. The tradeoff is ongoing maintenance, upgrades, and the risk that “just one more change” turns into a long engineering backlog.
Commercial enterprise LIMS can be very capable. In many cases, they are deployed in self-hosted forms, which can be a fit for certain IT policies, but it also brings infrastructure ownership and upgrade coordination that cloud buyers often want to avoid.
Cloud-based LIMS tends to win on speed of rollout and lower internal IT load. It can also support distributed teams better, because access and scaling are simpler.
Scispot typically fits that “move fast without losing control” category. It’s designed for quick configuration, clean data modeling, and integrations that keep workflows connected as labs scale.
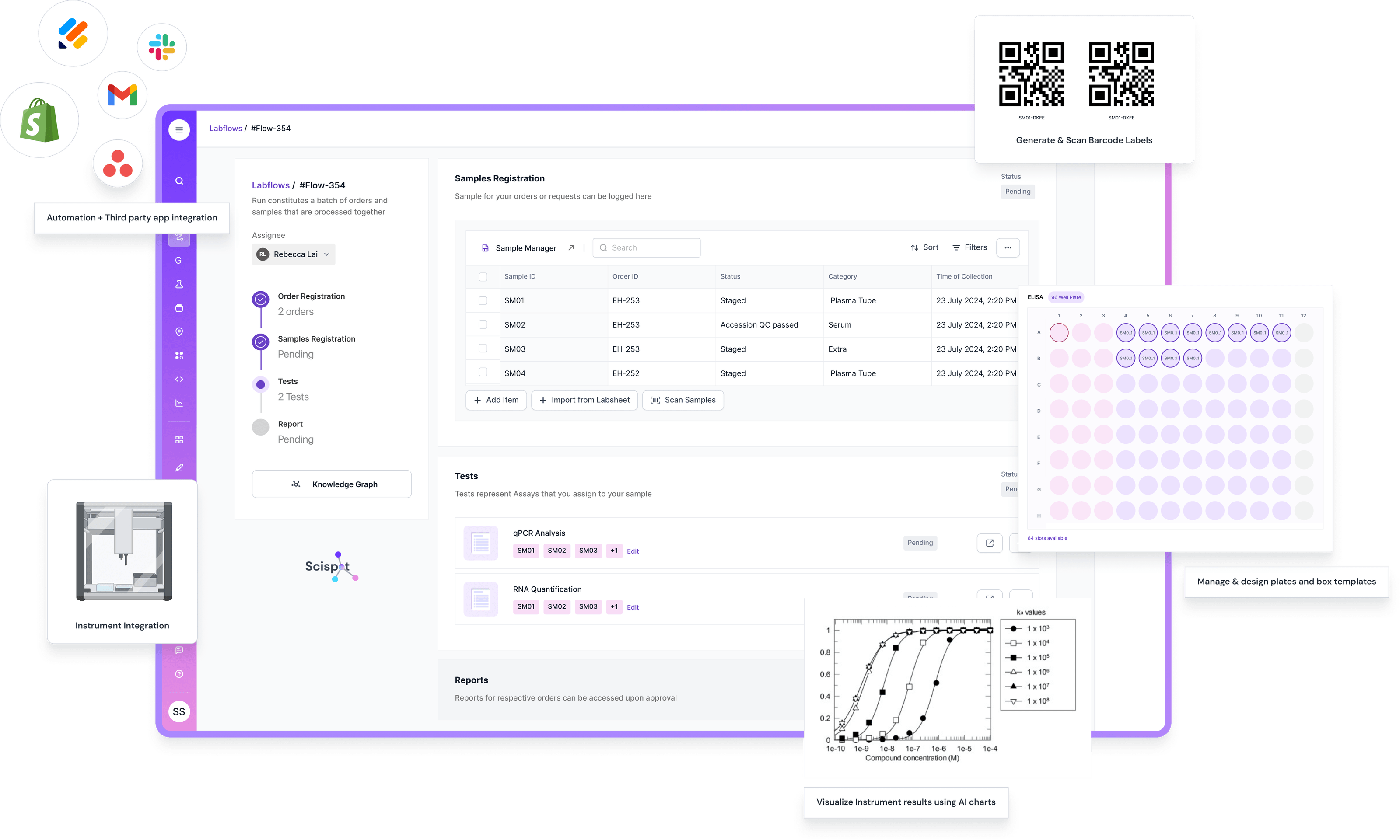
Scispot as a Modern Cloud LIMS in Practice
You can add a short “real-world example” block here, because you’ve already explained what a LIMS is, what it does, and what types exist. This is the natural spot to show what a modern LIMS looks like in practice. Scispot fits cleanly into the cloud-based LIMS bucket, but it also goes a step further by acting like a lab’s operating layer, not just a database. It keeps samples, results, workflows, and approvals connected, so teams spend less time stitching tools together and more time running science.
Scispot stands out when you map it back to the core LIMS features you listed earlier. Sample management stays tight with strong lineage from receipt to reporting. Workflow automation feels practical, because Labsheets and Labflows let teams model the way their lab actually runs, without forcing awkward workarounds. Integrations also become a first-class workflow, because GLUE can pull in instrument outputs and standardize them into structured fields, which improves data quality and reduces manual entry. Compliance work is easier too, because you can build around audit trails, e-signatures, access controls, and review steps in the same system where the data is created.
It also aligns with where LIMS is going next. If the future is AI-assisted analysis, real-time monitoring, and faster decisions, then the system needs clean, structured data and consistent workflows. Scispot’s dashboards and analytics layer make that feel less like a “data team project” and more like a daily lab habit. Think of it like moving from a filing cabinet to a live control panel, where the same data you capture for compliance also becomes usable for trends, QC signals, and operational planning.
LIMS Implementation: What to Consider
Implementation is rarely just “install software.” It’s process mapping, data cleanup, configuration, integration planning, validation needs, and training. The practical risk is drift. If requirements keep changing midstream, timelines and adoption can suffer, especially when the platform needs specialized expertise for every adjustment.
A strong sign you picked well is how quickly you can make safe changes after go-live. That’s where Scispot tends to shine, because teams can evolve Labsheets and workflows without turning iteration into a slow, expensive cycle.
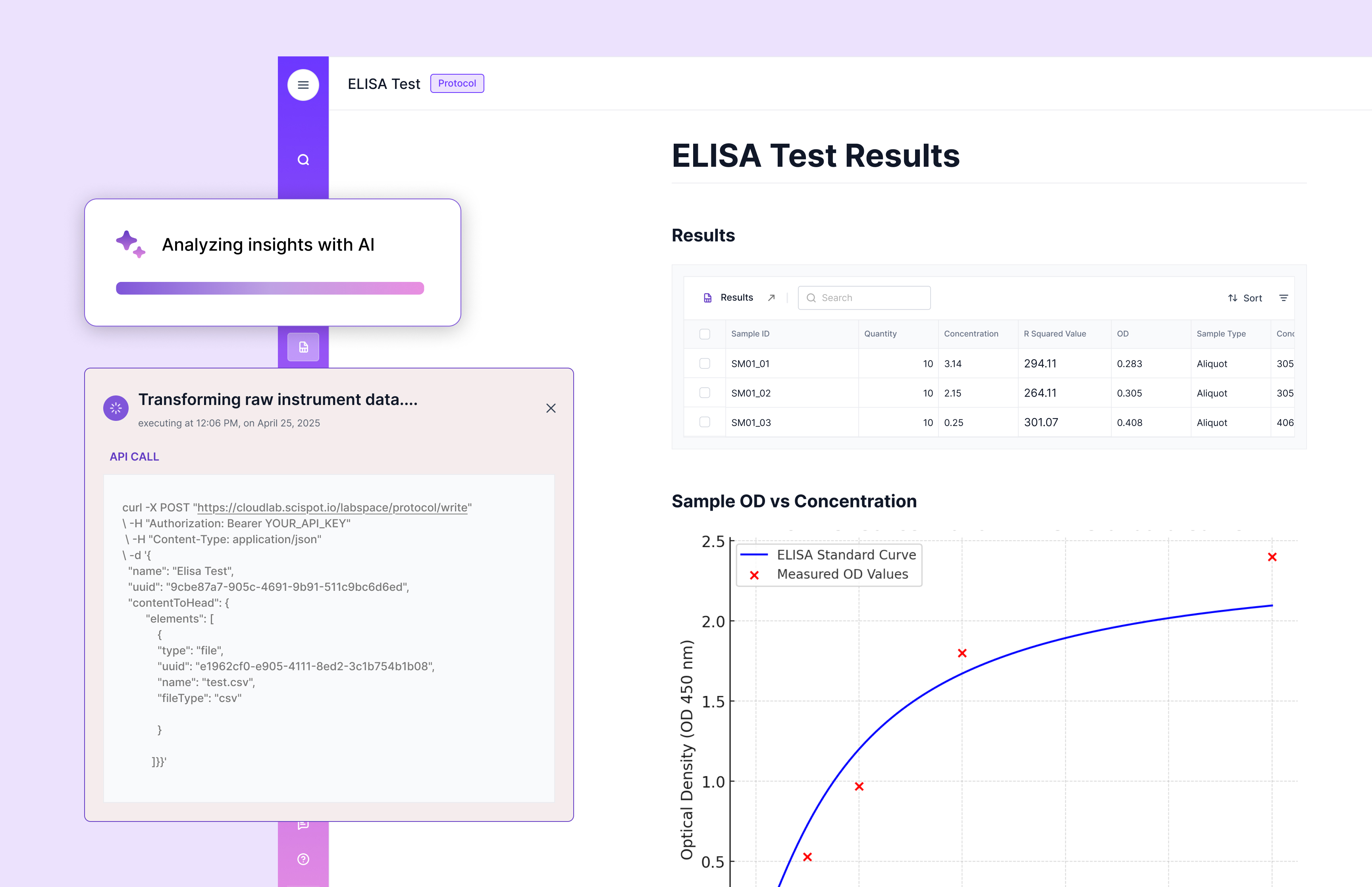
LIMS vs. Other Laboratory Management Tools
Basic lab tools can track a narrow slice, like inventory or task lists. LIMS is different because it connects samples, data, workflow, and approvals in one governed record.
The biggest gap is automation and integration. LIMS is designed to pull results in, standardize them, and route them for review, while simpler tools often leave labs stitching steps together by hand.
Scispot’s angle is that governance should not feel heavy. It aims to keep compliance-ready structure, while still feeling modern and fast for day-to-day users.
The Future of LIMS: Trends and Innovations
Cloud LIMS will keep winning because labs want easier scaling and less IT ownership. Teams also want upgrades and security patches to feel routine, not like a project that disrupts operations.
AI will shift from “dashboards later” to “decisions during work.” Think auto-flagging outliers, spotting drift, and nudging reviewers toward what needs attention first. Scispot fits this direction well because it’s built around structured Labsheets data and workflow context, which is what AI needs to be useful, not noisy.
Instrument connectivity will expand fast. More devices will push more files and more metadata. Labs will expect “connect, standardize, and trace” instead of export-heavy processes. Scispot’s GLUE layer is designed for that reality, so integrations can scale without turning every change in file format into a custom rebuild.

Conclusion: Why LIMS Matters in Modern Laboratories
LIMS matters because labs run on trust. Trust in sample identity. Trust in results. Trust in who changed what and when.
The best LIMS keeps that trust while still letting the lab move quickly. That usually means configurable data models, clean integrations, and audit-ready traceability that stays usable for day-to-day scientists. This is where Scispot tends to stand out, because it’s designed for fast workflow changes without losing control over lineage, reviews, and reporting.




.webp)
.webp)
.webp)
.webp)



