What is a Lab Audit?
Laboratory audits are an essential part of ensuring compliance with regulatory standards and maintaining efficient, safe operations. Whether preparing for a routine inspection or conducting an internal review, understanding the audit process and having the right checklists can make all the difference. This article outlines the key components of a lab audit, including essential checklists and preparation tips to streamline the audit process.
Lab audits play a critical role in maintaining the integrity of your laboratory’s operations. They help identify areas for improvement, ensure compliance with industry standards, and foster a culture of safety and quality. Regular audits can prevent costly non-compliance issues, improve the quality of results, and guarantee the overall safety of your laboratory environment.
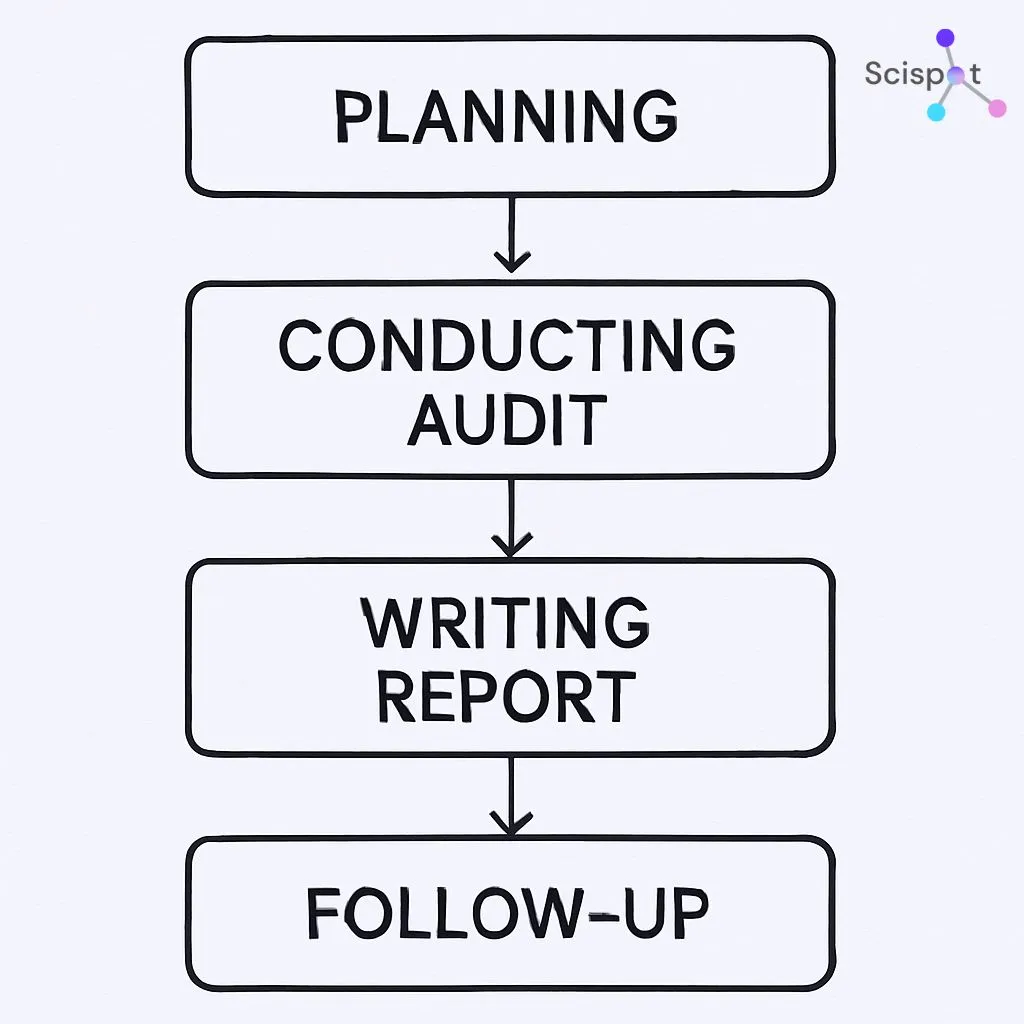
Key Components of a Lab Audit
Analytical Lab Audit Checklist
An analytical lab audit checklist ensures your lab meets the necessary standards for analytical testing. Here’s what a comprehensive checklist should include:
- Equipment Calibration and Maintenance: Ensure that all analytical instruments are calibrated and maintained as per manufacturer specifications. Scispot integrates seamlessly with lab instruments, making calibration tracking easier and more efficient.
- Standard Operating Procedures (SOPs): Verify that SOPs are up-to-date and accessible to all lab personnel. Scispot’s document management system ensures that the latest SOPs are always available and easily accessible.
- Data Integrity: Check that proper systems are in place for data recording, storage, and retrieval. With Scispot, you get built-in data traceability and audit trails that ensure data integrity without manual oversight.
- Sample Management: Review procedures for sample collection, labeling, and storage. Scispot streamlines sample tracking, ensuring every sample is accurately tracked from collection to analysis, reducing the risk of errors that may be common with older systems.

GMP Lab Audit Checklist
Good Manufacturing Practices (GMP) are essential for labs that support manufacturing processes. A GMP audit checklist should include:
- Facility Cleanliness: Inspect the cleanliness and organization of the laboratory environment. Scispot helps labs track and maintain cleanliness protocols with real-time updates and task management.
- Personnel Training: Confirm that all staff have received appropriate GMP training. Scispot’s training tracking feature ensures that personnel are always up-to-date with their compliance requirements.
- Documentation: Ensure all processes are well-documented and records are meticulously maintained. Scispot’s audit trails and version control capabilities ensure that documentation is always compliant and available for inspection.
- Quality Control: Evaluate quality control measures in place to ensure consistent results. Scispot’s automated QC checks reduce human error and ensure consistent quality in every batch of results.
Preparing for a Lab Audit
Preparation is key to ensuring a smooth audit. Here are a few tips:
- Inspection Readiness: Conduct internal audits regularly to address potential issues before an external audit. Scispot’s integrated system allows you to perform ongoing internal audits with minimal effort, ensuring your lab is always audit-ready.
- Document Review: Ensure all documents, such as SOPs, training records, and maintenance logs, are up-to-date and easily accessible. Scispot’s centralized document management system allows you to store and retrieve compliance-related documents at the touch of a button, reducing time spent on manual reviews.
- Staff Training: Train your staff on audit protocols, ensuring everyone understands their role in ensuring compliance and safety.

Audit Process Steps
- Notification: Receive the audit notification and understand the scope and criteria.
- Pre-Audit Meeting: Meet with your team to discuss the audit plan and address concerns.
- Audit Execution: Be present during the audit to facilitate the process and provide necessary documentation. Scispot’s audit trails make it easy to track every action, ensuring transparency and traceability.
- Post-Audit Review: Review audit findings and create a plan to address any identified issues. With Scispot, you can quickly track and document actions taken in response to audit findings, ensuring continuous improvement.
Specialized Lab Audit Checklists
Microbiology Lab Audit Checklist
Microbiology labs have specific requirements. Their checklist should include:
- Sterility Procedures: Verify procedures for maintaining sterility during sample handling and testing.
- Contamination Control: Ensure that systems are in place to prevent cross-contamination.
- Equipment Validation: Confirm that all microbiological equipment is validated and functioning properly.
ISO 13485 for Outsourced Testing Labs
For labs following ISO 13485 standards, the audit checklist should focus on:
- Quality Management System: Verify the implementation of a comprehensive quality management system. Scispot’s flexible workflows and integrated data management system ensure that quality is built into every stage of your operation, simplifying ISO 13485 compliance.
- Supplier Management: Review procedures for evaluating and managing suppliers.
- Risk Management: Assess risk management processes.
- Customer Feedback: Ensure there is a system in place for capturing and acting on customer feedback.
Ensuring Lab Safety
Lab safety is paramount in any audit. A safety checklist should include:
- Personal Protective Equipment (PPE): Confirm that appropriate PPE is available and being used by all personnel.
- Emergency Procedures: Ensure that emergency procedures are visible and understood by all staff.
- Chemical Storage: Verify proper labeling and storage of chemicals.
- Fire Safety: Confirm that fire extinguishers and safety showers are easily accessible.
The Scispot Advantage
Unlike many legacy systems, Scispot provides real-time monitoring, integrated data flows, and automated tracking, ensuring that your lab can perform audits seamlessly. Other platforms often require manual updates and patchwork integration, which can lead to inconsistencies in compliance and increase the risk of human error. With Scispot, all compliance-related processes are centralized, making audits a more efficient and effective experience.
By integrating Scispot’s LIMS and ELN, labs can easily manage sample tracking, instrument calibration, data analysis, and regulatory compliance under one unified platform. This holistic approach is something that older systems, such as LabWare and Matrix Gemini, struggle to deliver without complex and expensive integrations.
Conclusion
Lab audits are crucial for maintaining compliance and enhancing the safety and quality of laboratory operations. With the right tools in place, audits become an opportunity for growth, ensuring your lab is always ready for the next challenge. Scispot’s LIMS and ELN solutions support this process by simplifying audit preparation, maintaining data integrity, and fostering continuous improvement across all areas of your lab’s operation. Stay proactive, leverage Scispot’s powerful features, and ensure that your lab is always ready for the next audit, enhancing both productivity and regulatory compliance.
.gif)
By making the right investment in Scispot, labs can reduce their time spent on audits and focus on their primary mission: driving innovation and achieving scientific breakthroughs.




.webp)
.webp)
.webp)
.webp)



