Maintaining United States Food and Drug Administration–grade data integrity for large, analytics-driven image collections requires more than secure storage. It requires computer-generated, time-stamped audit trails, standards-based provenance that ties pixels to results, and a validation approach that stands up in inspection. Scispot delivers these pillars through controls aligned to Title 21 of the Code of Federal Regulations Part 11 for electronic records and signatures, the ALCOA+ data-integrity principles, open imaging standards such as Open Microscopy Environment Tagged Image File Format and Open Microscopy Environment Zarr, and a validation toolkit compatible with Good Automated Manufacturing Practice 5 Second Edition. These capabilities operate within a security posture that includes System and Organization Controls (SOC) 2 Type I and are hosted on an International Organization for Standardization (ISO) 27001–certified infrastructure.

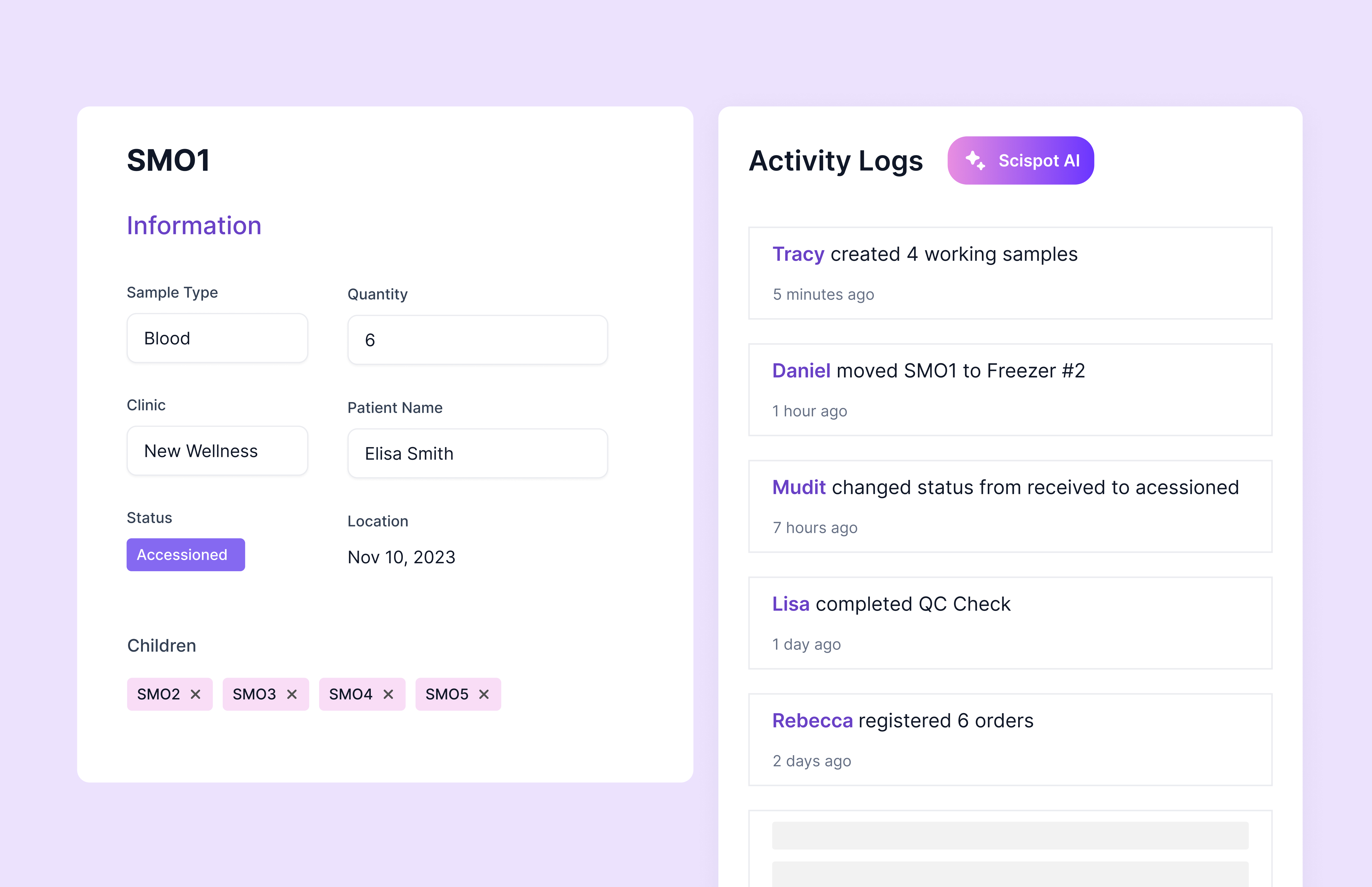
Scispot captures computer-generated, time-stamped audit entries for each material action on regulated records. Entries preserve who did what, when, and how the record changed, so prior values are never obscured. Electronic signatures record the signer’s identity, purpose, and time. Together, these controls satisfy the Part 11 requirement for secure, time-stamped audit trails, and they operationalize ALCOA+ by keeping data attributable, legible, contemporaneous, original, accurate, complete, consistent, enduring, and available. To make audit chains tamper-evident over the full retention window, Scispot can be deployed with Write Once Read Many retention using Amazon Web Services Simple Storage Service Object Lock in Governance or Compliance mode, with retention periods set in the quality management system.

Provenance starts at acquisition and continues through analysis. Scispot’s Graphical Linking and Unification Engine ingests and registers imaging artifacts in OME-TIFF or OME-Zarr, and binds them to structured metadata, including instrument identifiers, acquisition settings, timestamps, algorithm names and versions, and parameter sets. That linkage creates a deterministic path from source pixels to derived results, enabling reproducibility and re-analysis. Images remain in vendor-neutral containers, allowing teams to continue analyzing or visualizing data in their preferred external tools while Scispot preserves the chain of custody across samples, plates, and studies.
Validated operation is addressed through documentation and life-cycle practices recognized by regulators and auditors. Scispot supports Computerized System Validation with user and functional specifications, traceability, and installation, operational, and performance qualification evidence aligned to Good Automated Manufacturing Practice 5 Second Edition. Security controls include a System and Organization Controls 2 Type I report, hosting on Amazon Web Services facilities certified under ISO 27001, encryption in transit per National Institute of Standards and Technology Special Publication 800-52 Revision 2 using Transport Layer Security 1.2 or 1.3, and encryption at rest consistent with National Institute of Standards and Technology Special Publication 800-175B guidance for Advanced Encryption Standard. These measures support vendor-risk assessments and inspection readiness.
Interoperability is delivered through an application programming interface–first design and managed connectors. Scispot’s Graphical Linking and Unification Engine integrates instruments and software, including electronic laboratory notebooks and laboratory information management systems, so validated image packages and machine-readable provenance can be archived to quality or document systems on schedule. This reduces manual transfers, preserves context, and shortens inspection prep.
A typical inspection scenario looks like this. A quality steward exports an audit log from Scispot for a completed whole-slide assay and sees Coordinated Universal Time timestamps, operator identifiers, operation types, and old-to-new value changes with electronic approvals. The steward queries Scispot’s Graphical Linking and Unification Engine to confirm that each derived metric references the algorithm name, version hash, and parameter set. The associated OME-Zarr and OME-TIFF artifacts are under Amazon Simple Storage Service Object Lock in Compliance mode for seven years, matching the site’s retention policy. The steward retrieves the Good Automated Manufacturing Practice–aligned validation bundle with specification traceability and installation and operational qualification evidence, and then triggers an export through the application programming interface to the quality management repository. The lab now holds both human-readable inspection reports and machine-verifiable lineage for every image-driven result.
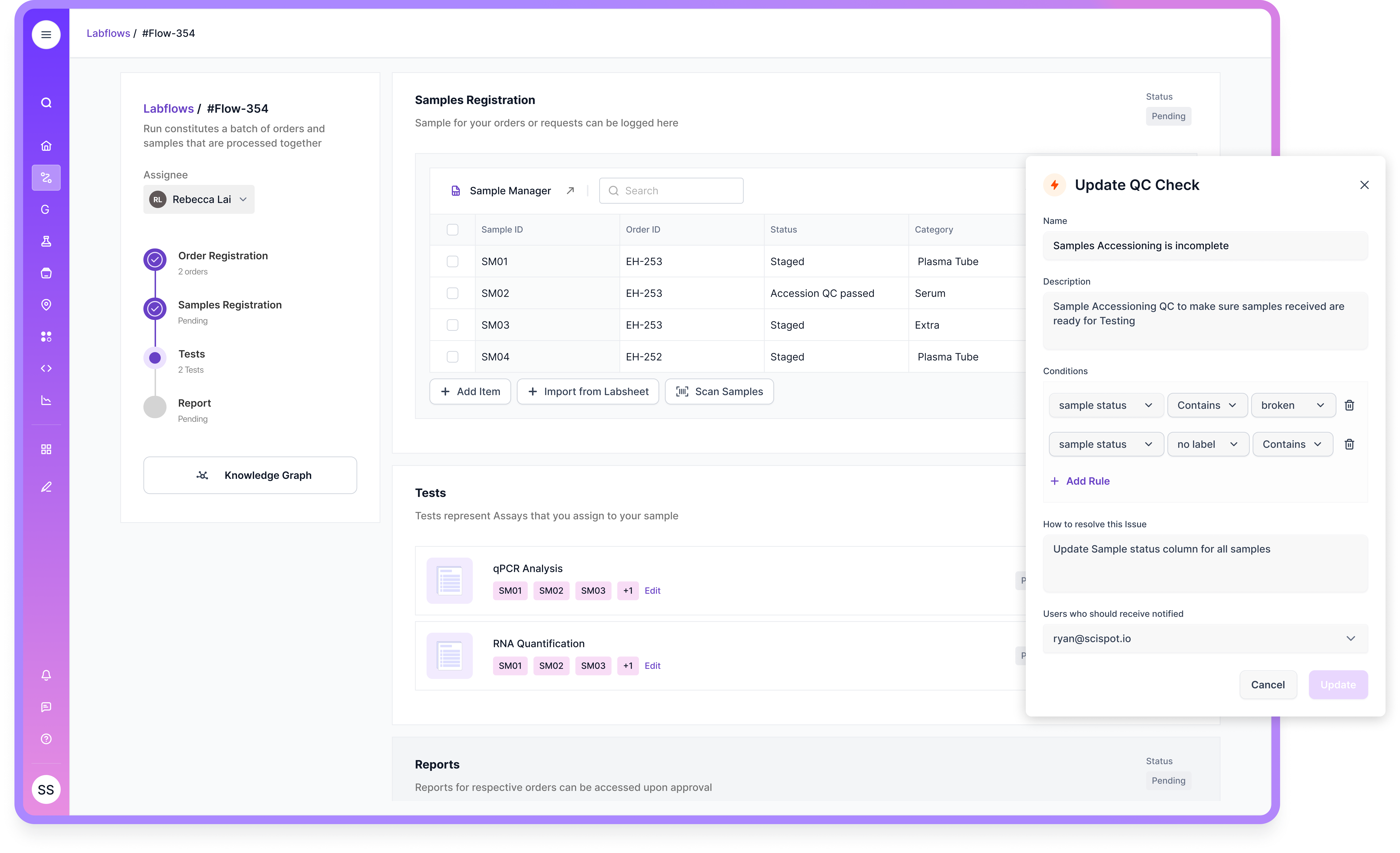
If your procurement team needs proofs, Scispot can supply sample audit-trail exports with ISO-8601 timestamps and reason-for-change fields, demonstrate registration of Open Microscopy Environment packages with sidecar JavaScript Object Notation provenance, provide the current System and Organization Controls 2 Type I report and hosting attestations, and deliver the validation bundle mapped to Good Automated Manufacturing Practice 5 Second Edition. These artifacts convert compliance from a manual burden into a repeatable, inspectable workflow embedded in daily operations.






.webp)
.webp)
.webp)



