When a nonprofit research institute set out to build a more open, connected future for early drug discovery, its biggest challenge wasn’t the science—it was the system behind it.
Despite the brilliance of its scientists and collaborators, day-to-day lab work had become buried under spreadsheets, scattered files, and disconnected tools. Experiments lived in one place, instrument data in another, and reports took hours to compile. It wasn’t sustainable for a team built on the ideals of open science and reproducibility.
That’s when they turned to Scispot.

Scispot wasn’t just another software platform—it became their bridge between curiosity and compliance, between discovery and delivery. The first thing that struck them was how intuitive it felt. Within two weeks, scientists who once dreaded digital systems were running stepwise experiments, uploading results directly from instruments, and signing off on records with ease.
It started with structure. SOPs that once sat in Word documents were transformed into dynamic workflows. Every run could be tracked, reviewed, and locked after completion, leaving behind a clean, auditable trail. There were no more “Who edited this?” moments or lost versions of results. Scispot brought order without slowing them down.
Instrument data, which used to arrive as messy files from qPCRs, HPLCs, and plate readers, now flowed seamlessly into the system. For a small team, that mattered—a lot. There was no IT department to manage complex integrations. Scispot’s managed setup let them start with simple file uploads and evolve into automated data ingestion once they were ready. It was flexible, not forced.

Then came visibility. Equipment bookings stopped overlapping. Managers could open their dashboards and see exactly who was running what, where, and when. Scientists didn’t need to ask around to know which instrument was free—they could just check and go. For a team working across multiple programs and collaborations, that clarity meant everything.
Perhaps the biggest shift came with compliance. Scispot introduced training-gated permissions—access to edit or run certain workflows wasn’t granted by default; it had to be earned through completed training. It created a culture of accountability that felt empowering rather than restrictive. Scientists knew they were working within guardrails that protected data integrity and trust.
And when reports were due—those critical Certificates of Analysis (COAs) that once consumed entire afternoons—they could now be generated in minutes. Branded, locked, and fully traceable. It wasn’t just about efficiency; it was about pride. Their data looked as good as their science.
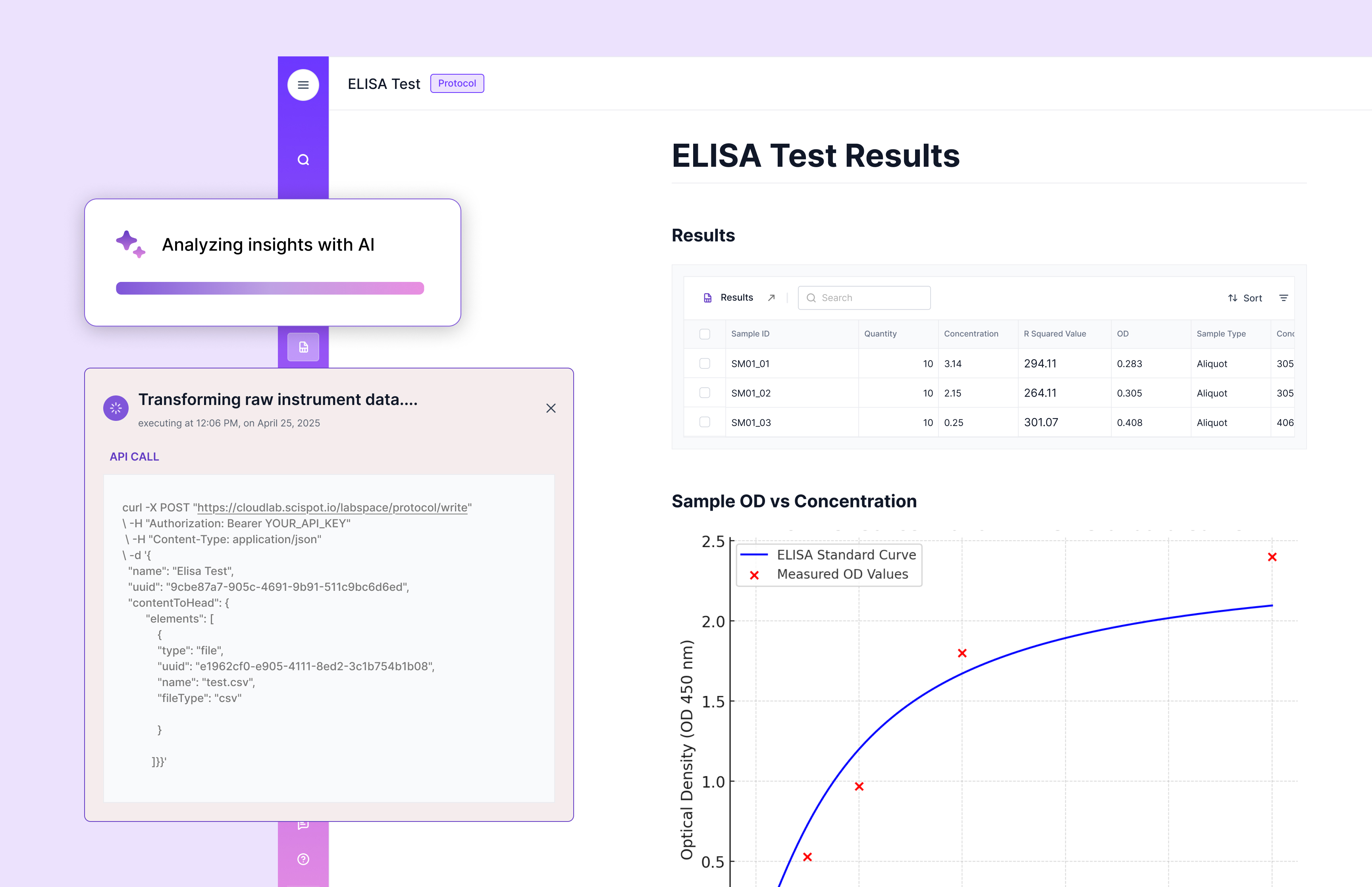
The transformation didn’t happen overnight, but it didn’t take long either. In under 90 days, this institute went from fragmented systems to a unified, compliant, and collaborative Lab Operating System. Their scientists stopped chasing data and started chasing discoveries again.
For organizations that live at the intersection of science and collaboration, Scispot is more than a digital platform—it’s the quiet infrastructure that lets brilliance flow freely.




.webp)
.webp)
.webp)



