What are common issues found during lab audits and how to fix them?
Lab audits are one of the fastest ways to spot silent risk inside a lab. They uncover gaps that can quietly break compliance, slow down results, or weaken data integrity. Audits can be internal or external, and they usually map back to standards like ISO 17025, CLIA/CAP, GLP, GMP, or FDA expectations.
.webp)
Lab audits are systematic checks of lab processes, records, and controls. They confirm whether the lab is following its own procedures, maintaining traceability, and protecting data. A lab can be doing excellent science and still get flagged if the proof of work is scattered, incomplete, or hard to verify.
Common Issues Found in Lab Audits
Inadequate Documentation
This is one of the most frequent audit findings across labs. Records exist, but they are often spread across spreadsheets, paper binders, PDFs, and shared drives. That fragmentation makes it hard to show the full story behind a result, especially when auditors ask for who performed an action, when it happened, what changed, and why it was changed.
In many labs, documentation becomes “extra work” done after the experiment. That’s where errors happen. A modern LIMS like Scispot makes documentation a natural part of execution. When test runs, sample activity, deviations, and approvals are captured in structured templates, audit-ready records become a byproduct of daily work.
Non-compliance with Standard Operating Procedures (SOPs)

SOP gaps often show up when SOPs live as static documents and execution happens in real life under time pressure. Small deviations add up over time. Teams start following “the way we do it” instead of “the way it’s documented,” and audits catch this mismatch quickly.
Older and rigid systems can make SOP execution feel like a checklist exercise, which leads to workarounds. Scispot supports SOPs as configurable workflows with required fields, checkpoints, and review steps. That keeps processes consistent without making scientists feel boxed in, and it reduces the chance of SOP drift as teams scale.
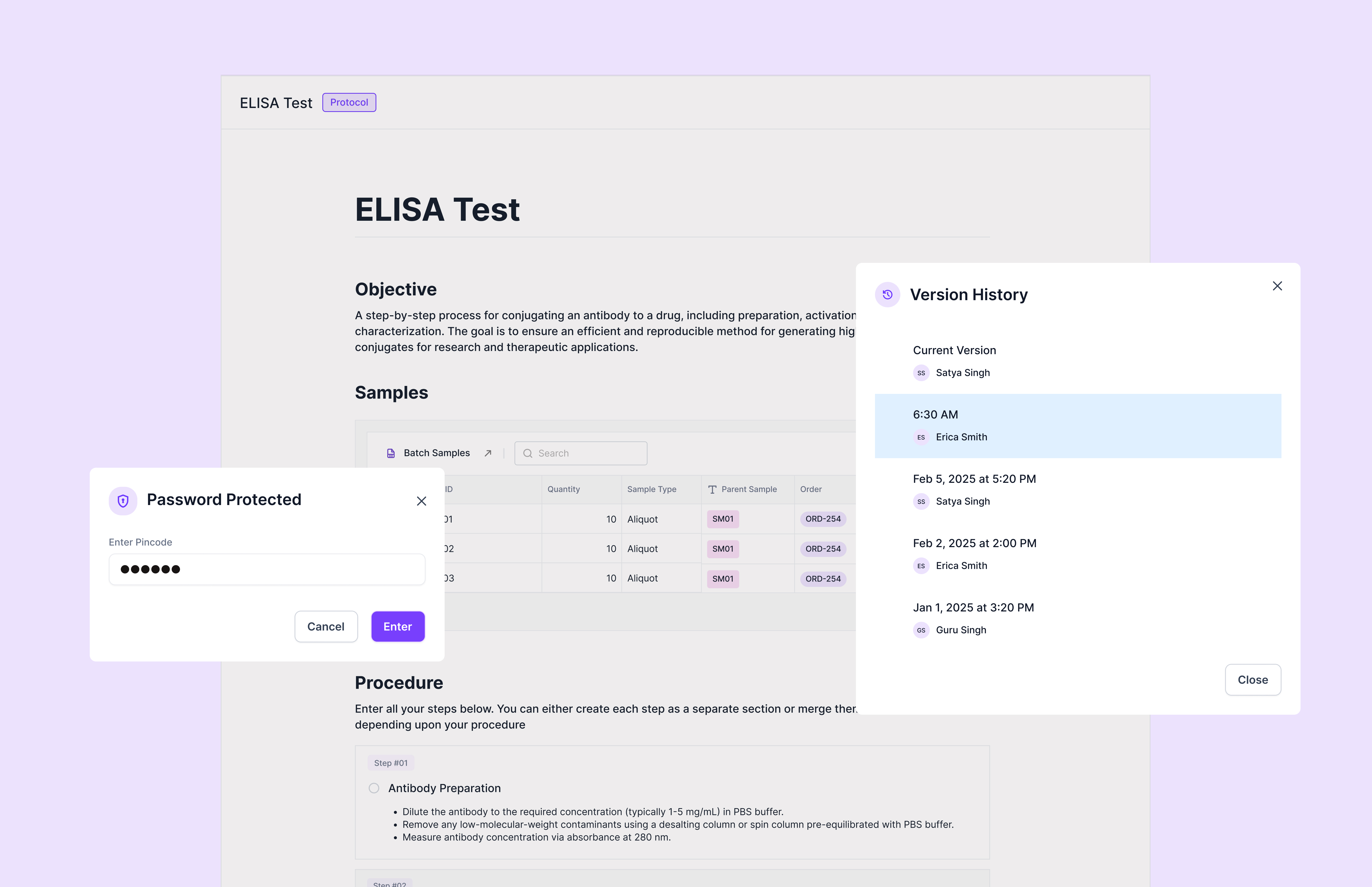
Security Issues
Security audit findings often come from unclear access control and weak accountability. Common examples include shared logins, broad permissions, missing evidence of review, and limited visibility into who accessed or changed key records. Even a well-run lab can get flagged if access policies are not enforced consistently.
Some teams try to solve security by layering tools on top of their workflow, but that usually creates more gaps. Scispot keeps security tied to operations through role-based access, controlled reviews, and traceable activity history. That makes it easier to show auditors that the right people had the right access at the right time.
Equipment Calibration and Maintenance
Calibration and maintenance issues are easy for auditors to identify. They show up as missing certificates, overdue schedules, incomplete logs, or unclear evidence that an instrument was within calibration when a test was run. This becomes serious because equipment validity directly impacts the reliability of results.
In many labs, equipment tracking sits outside the testing process, which makes it easy to miss. Some legacy platforms support equipment modules, but they can be heavy to maintain and harder for teams to use day-to-day. Scispot makes equipment readiness easier to operationalize by keeping calibration logs, reminders, and usage traceability close to the workflows that depend on them.
Training Deficiencies
Training findings are rarely about “no training.” They are more often about missing proof, outdated content, inconsistent competency tracking, or gaps when SOPs change. Auditors want clean evidence that staff were trained, qualified for their roles, and re-trained when procedures evolved.
Training often breaks down when it’s managed in spreadsheets and shared folders. Scispot makes training easier to standardize by supporting versioned workflows, structured reviews, and traceable changes over time. That helps labs show a clear timeline of what changed, who approved it, and who was trained on it.

How Scispot Helps You Fix Audit Issues Faster
Most audit issues don’t happen because teams don’t care. They happen because lab work is spread across spreadsheets, folders, emails, and disconnected tools. Documentation gets missed, SOP steps drift, and training proof becomes hard to track. Scispot helps by bringing your lab’s records, workflows, and approvals into one structured system that stays consistent even when the lab is busy.
For documentation and SOP compliance, Scispot keeps every record clean and traceable. It captures audit trails automatically, tracks version history, and supports electronic signatures and role-based access. So when an auditor asks “who changed this result, when, and why,” your team can answer in seconds instead of hunting through old files.
For equipment and training gaps, Scispot links calibration logs, maintenance history, and instrument usage directly to the work being done. It can send reminders for upcoming calibration, flag overdue equipment, and store proof of competency for every staff member. That means audits become more predictable, and your lab builds a habit of staying ready instead of rushing at the last minute.
How to Fix Common Audit Issues
Improving Documentation Practices
The fastest way to improve audit outcomes is to treat documentation like a system, not a habit. You want consistent fields, controlled templates, clear ownership, and built-in traceability. When a lab depends on memory and manual updates, documentation will always lag behind real work.
Scispot helps by ensuring documentation is captured during execution, not after. Test data, sample movements, approvals, and change history stay connected in one place. That makes it easier to retrieve records quickly during audits and reduces the stress of last-minute evidence gathering.
Ensuring SOP Compliance
.jpg.webp)
SOP compliance improves when SOPs are embedded inside the workflow itself. That means required fields, enforced checkpoints, and review steps that happen as part of the process. When SOPs are separate from execution, teams naturally drift, especially during busy cycles.
Many older systems create friction through rigid workflows and complex interfaces, which increases the likelihood of side spreadsheets and informal shortcuts. Scispot keeps SOP execution practical by allowing labs to configure workflows that match real lab operations while still enforcing compliance controls. This keeps teams consistent without slowing them down.
Enhancing Security Measures
Security should be enforceable, visible, and provable. Auditors look for access control, strong accountability, and clear evidence of review and approval. If it’s not traceable, it might as well not exist during an audit.
Scispot supports role-based access controls and structured review flows so that sensitive actions require the right permissions. This reduces the risk of unauthorized edits and creates a clean record of who did what, when, and under what approval context.
Regular Equipment Maintenance and Calibration
Equipment programs work best when they are predictable and hard to miss. Labs need scheduled routines, automated reminders, and complete logs that can be pulled instantly during an audit. The strongest setups link equipment state to actual test runs, so it’s easy to prove that results came from validated instruments.
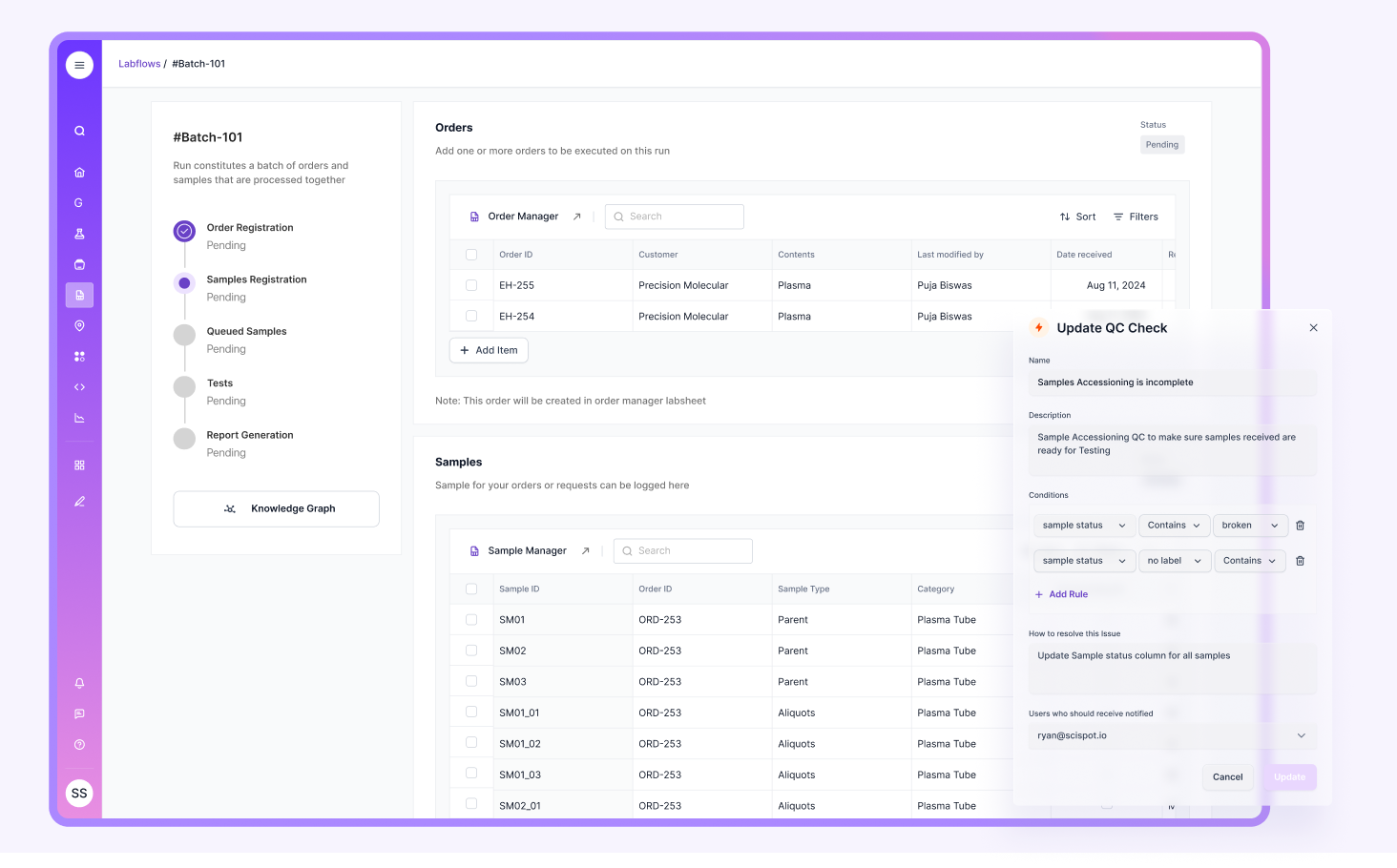
Many labs fall into the trap of tracking maintenance separately from lab execution, which creates blind spots. Scispot keeps equipment tracking connected to workflows, making it easier to maintain readiness and prove calibration status without extra manual effort.
Strengthening Training Programs
Training becomes audit-ready when it is repeatable and measurable. You need proof of completion, proof of role qualification, and proof that the training stayed current. A training program should also evolve smoothly as SOPs evolve, so new changes don’t create hidden gaps.
Scispot supports structured processes with versioning and review controls that make training easier to update and track. This allows labs to show a clear, evidence-backed story to auditors, including what changed, when it changed, and how the team stayed aligned.
Conclusion
Lab audits don’t just assess compliance. They reveal where labs are most likely to break under scale, turnover, or increased regulatory pressure. The most common failures usually come back to documentation quality, SOP drift, security gaps, equipment readiness, and inconsistent training evidence.
Some older LIMS platforms are feature-rich, but they often bring usability friction and complex setup, which pushes teams toward side tools like spreadsheets and PDFs. That patchwork is exactly what auditors tend to flag. Scispot keeps labs audit-ready by making compliance a natural part of daily work through structured workflows, traceable records, and connected systems that scale with the lab.
.gif)
When labs address these gaps proactively, audits become far less stressful. They also build stronger foundations for quality, data integrity, and operational speed. With the right process design and a modern LIMS like Scispot supporting execution, labs can move from “audit survival mode” to true audit confidence.




.webp)
.webp)
%20(1).webp)
.webp)



