What are the key modules in a LIMS?
In today’s fast-paced scientific environments, managing laboratory information well is not optional anymore. Labs need speed, traceability, and clean data. Laboratory Information Management Systems (LIMS) help streamline operations, reduce manual errors, and keep work audit-ready. This article breaks down the key modules that make a LIMS useful in modern labs, and what to look for when choosing one.

A LIMS is built to manage samples, associated data, and the processes that move work from request to result. It creates a single source of truth across people, instruments, and workflows. That matters in pharma, food testing, clinical research, and any environment where results must be trusted and repeatable. A strong LIMS does more than store data. It shapes how work happens, and how fast a lab can scale.
Why Scispot maps cleanly to modern LIMS needs
Many LIMS platforms cover the same “module checklist.” The real difference shows up in how connected those modules are, and how fast teams can configure them without turning every change into an IT project. Scispot is designed around modular building blocks that stay linked: structured data capture (Labsheets), workflow traceability (Labflows), and integration infrastructure (GLUE). This makes it easier for labs to move from “tracking work” to “running work” in one system, while staying compliance-ready.
A common friction point with many older, enterprise-style LIMS is time-to-value. Review sites often show long implementation cycles for certain legacy systems, and labs end up carrying spreadsheets and side tools during rollout.
Core modules of a LIMS
LIMS systems are highly configurable. Most labs start with a few core modules, then expand as volume, compliance requirements, or automation needs increase. The key is not just having modules. The key is having modules that share the same data model, so sample records, results, audit logs, and reports stay connected.
Sample management module
This is the heart of any LIMS. It tracks the full lifecycle of samples, from intake to storage to testing to disposal. Good sample management supports barcodes, chain-of-custody, status tracking, and fast search. In real labs, this module prevents “lost samples,” mixed IDs, and unclear ownership of work.
In Scispot, sample tracking is not isolated from the rest of the system. Samples can link directly to structured result tables, instrument outputs, and workflow steps. That means the sample record is not just a label. It becomes the hub for context, results, and decisions.
ELN integration

ELN capability is often needed because labs do not only run tests. They also document methods, observations, deviations, and reasoning. Many vendors bolt ELN-like note-taking onto a LIMS. That can work for basic documentation, but it often breaks down when teams want structured, queryable experimental data that can be compared across runs.
Scispot’s approach leans into structured capture first. Labsheets are designed to store experimental and operational data in tables that are easy to search, filter, review, and report on. That also makes downstream analysis and dashboards far easier, because the data is already shaped for computation.
Instrument integration
Instrument integration is where many labs lose time. A system can look complete on paper, but still rely on manual file uploads and copy-paste from instrument software. That increases error risk, and it slows review.
Scispot GLUE is built as the integration and data backbone, so labs can connect instruments, flat files, and other tools, then standardize data into the same model used by Labsheets and workflows. This helps labs move from “data collection” to “data flow.”
It is also worth calling out a practical tradeoff seen across the market. Even vendors themselves acknowledge that heavily customized LIMS deployments take longer to configure, validate, and deploy. That delay often shows up when integrations and workflow changes require deep customization rather than configuration.
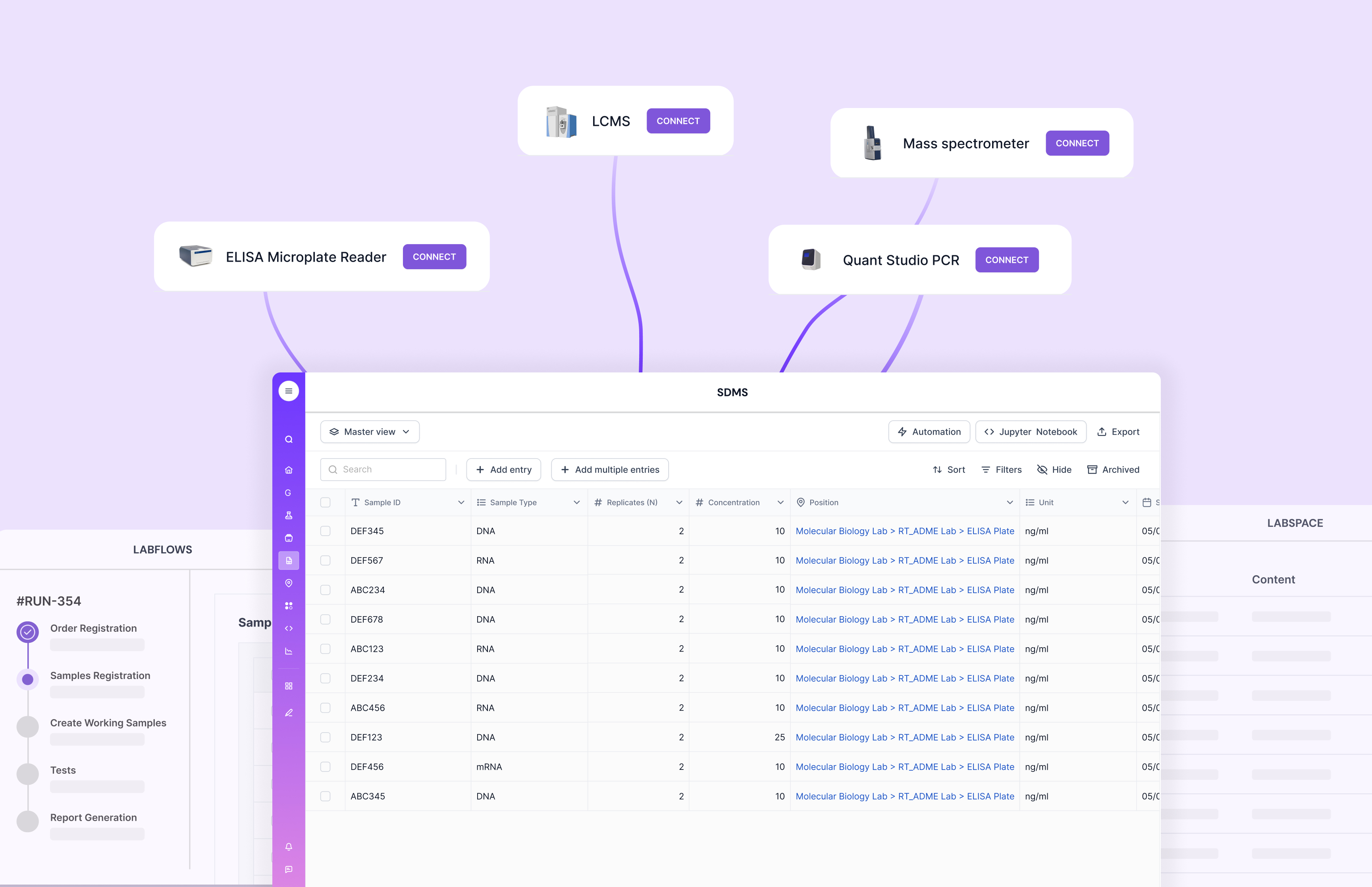
Workflow management
Workflow management keeps lab execution consistent. It helps define SOP steps, status gates, assignments, approvals, and dependencies. Without it, teams end up managing schedules and next steps in someone’s head, or in a separate tracker.
Scispot Labflows is purpose-built for end-to-end traceability. It links “what happened” to “who did it,” “on which sample,” “using which method,” and “with what result.” That makes quality review faster, and it makes investigations less painful when something goes wrong.
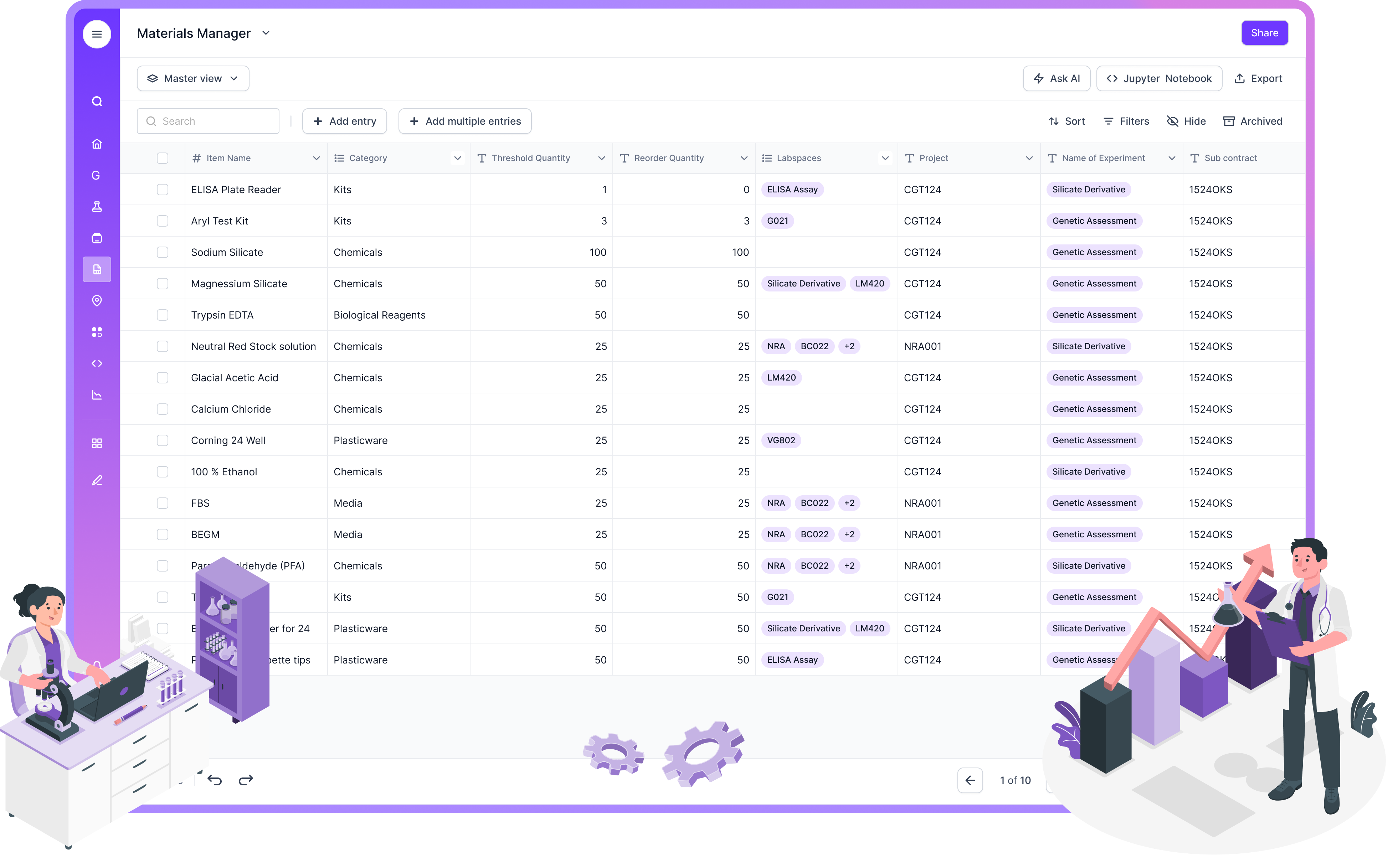
Inventory management
Inventory modules track reagents, consumables, lots, expiry, storage location, and usage. The impact is bigger than “not running out.” It supports repeatability. It supports compliance. It supports cost control.
In Scispot, inventory is not treated as a separate tool. It can be tied directly to samples, test runs, workflows, and results tables. That helps labs answer questions like “which lot did we use for these results?” without stitching together multiple systems.
Advanced modules that become critical as labs scale
As labs grow, the “nice-to-have” modules become essential. This is where teams start caring about audit depth, review cycles, analytics, and environment controls. It is also where older systems can feel heavy, because expanding capability often means adding more admin complexity and training.
Quality management
Quality modules help manage deviations, CAPA, controlled documents, and audit trails. These features reduce risk in regulated work. They also improve internal accountability in research settings.
Scispot emphasizes auditability and permissions as native capabilities. The goal is to make review and traceability feel like part of the workflow, not a separate compliance exercise.
Data analytics and reporting
Reporting is where many LIMS become frustrating. You may get fixed reports, or reports that need heavy consulting work to change. Labs then export to Excel anyway, which breaks traceability.
Scispot leans into structured data capture so reporting becomes simpler. When results are stored in tables with consistent schemas, dashboards and queries become easier to standardize across studies and sites.
Compliance and regulatory module
.jpg.webp)
This module helps labs adhere to local, national, and international regulations. It supports controls like audit trails, e-signatures, role-based access, and review history. This helps labs align with frameworks such as ISO, GLP, and FDA expectations.
When evaluating vendors here, watch for the difference between “supports compliance features” and “makes compliant workflows easy to run every day.” The second one is what reduces real operational risk, because people actually follow the system when it feels natural. Scispot positions security, permissions, and audit trails as built-in, rather than add-ons.
Environmental monitoring
Some labs must track temperature, humidity, and other conditions. The goal is not just monitoring. The goal is linking environment logs to storage, samples, and results. That makes investigations faster when stability issues show up.
A modern LIMS should make it straightforward to connect monitoring data to impacted materials and runs. In practice, this is another area where integrations matter, because the data often lives in separate systems.
Customizing a LIMS for your lab
No two labs run the same way. A good LIMS should let you configure data fields, workflows, templates, and permissions without turning every change into a software project. This is where the gap between configuration and customization matters. Configuration means you can adapt the system with admin tools. Customization often means longer timelines, more validation work, and more dependency on specialists.
Scispot’s value here is the way modules stay connected. If you change a schema in your structured data capture, it still links cleanly to workflows and integrations. That reduces “tool sprawl,” because teams do not need separate systems for execution, results, and traceability.

Choosing the right LIMS provider
Scalability matters, but so does rollout speed. If implementation drags, labs often keep parallel spreadsheets, which creates a split brain. Some platforms show long implementation averages in public review aggregates, which is a useful signal to ask tougher questions about time-to-value.
Support and training also matter more than buyers expect. A system can be powerful, but still require deep training to use well. Some vendors even highlight multi-day end-user training programs, and acknowledge that lack of training can set implementations back. That is not automatically bad, but it is a real cost in time and adoption risk.
Cost is not just licensing. It is also the cost of time, internal effort, validation, and the “hidden tax” of workarounds. Integration capabilities are the final filter. If the LIMS cannot connect cleanly to instruments and the rest of your stack, you will keep exporting files and reformatting data, which cancels out the benefit of having a LIMS at all. Scispot’s GLUE positioning is designed around that reality.
Conclusion: The future of LIMS
LIMS keeps evolving, because labs keep evolving. The direction is clear. Labs want fewer tools, faster onboarding, better integrations, and more usable systems. They also want strong compliance foundations without sacrificing day-to-day speed.
A practical way to think about it is this. A traditional LIMS can feel like a “vault.” It stores things safely, but it can be slow to move things in and out. Scispot aims to feel more like a “connected lab workspace.” Data capture, workflows, and integrations are designed to work as one system, so teams spend less time moving data and more time using it.
If you align LIMS modules to your real lab bottlenecks, the system becomes a force multiplier. It improves accuracy. It improves traceability. It makes growth less painful. And when the platform is modular and integration-ready, you can keep adding capability without rebuilding your foundation.




.webp)
.webp)
.webp)
.webp)



